TRUSTED BY
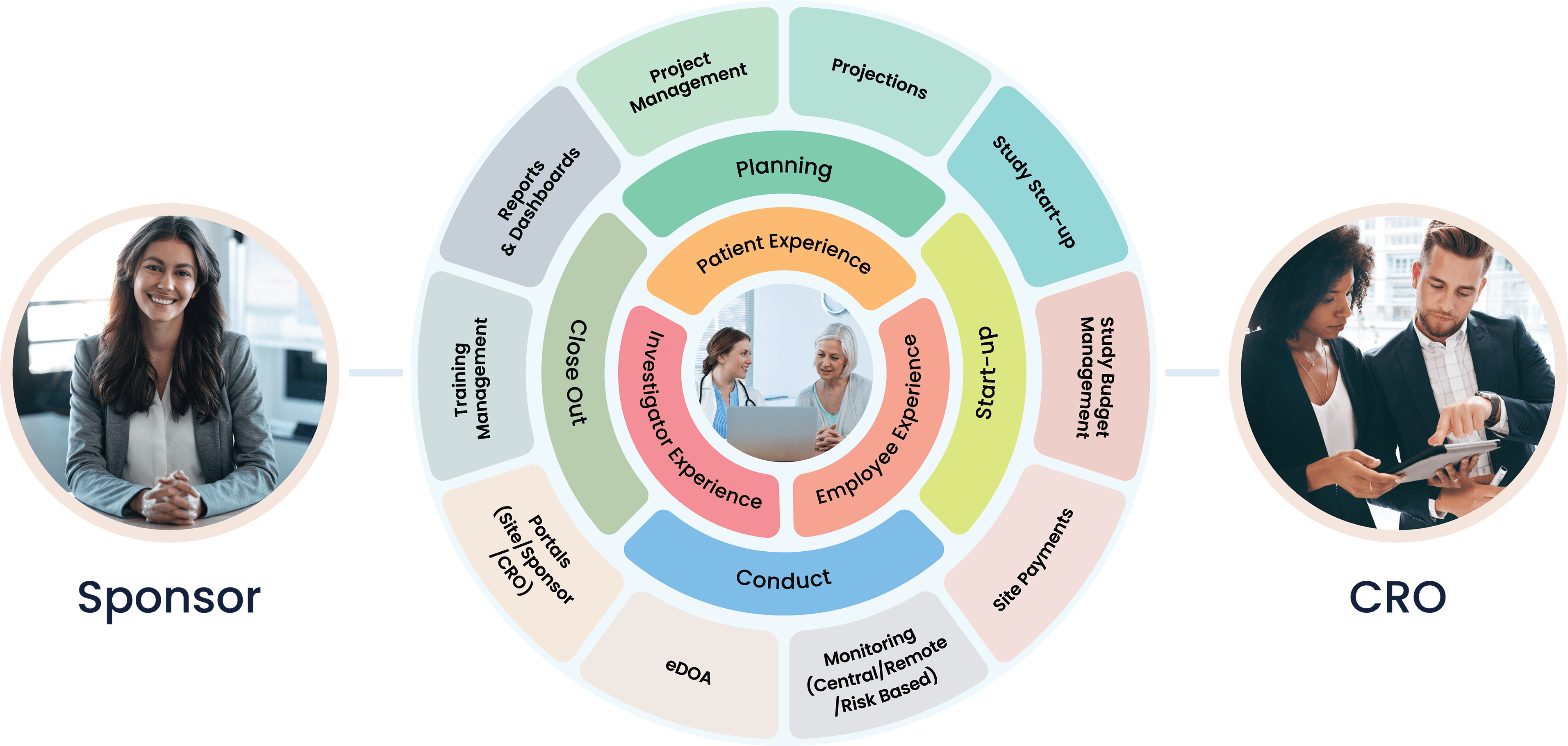
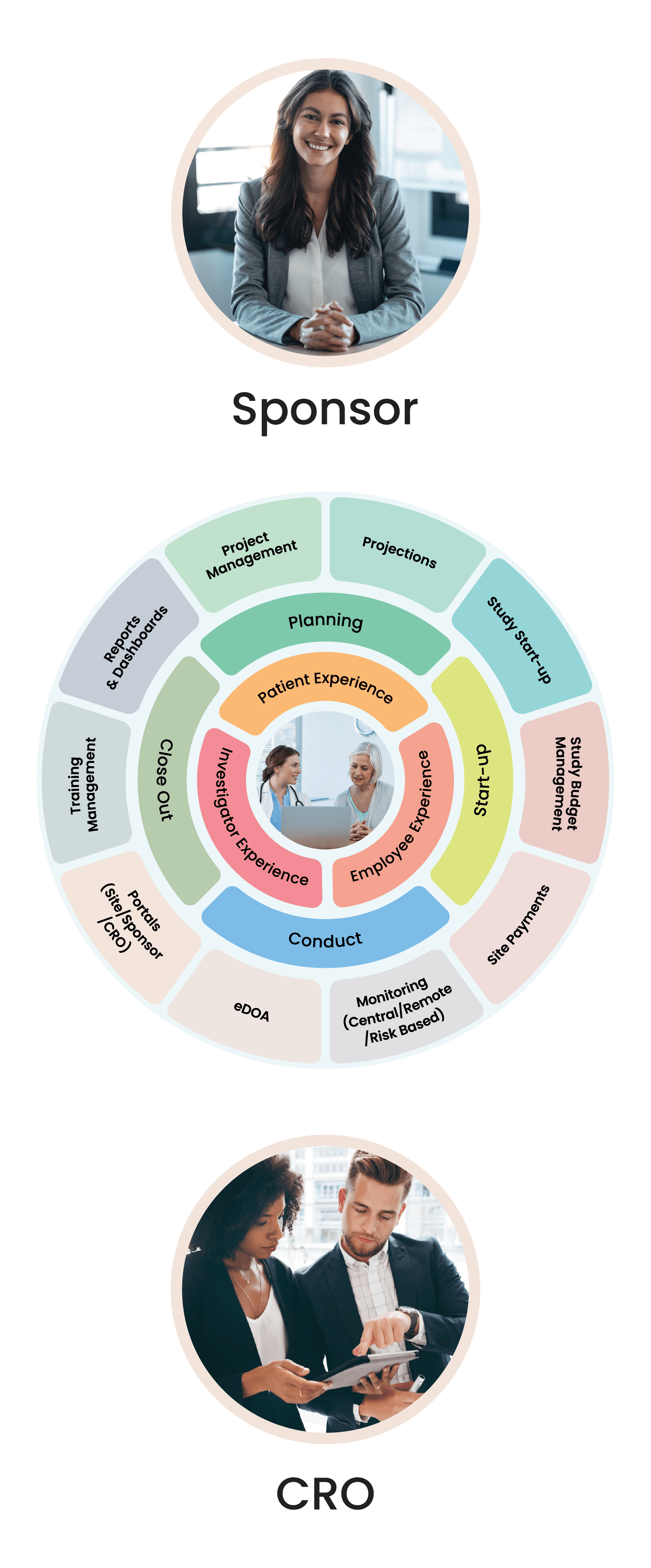
Cloudbyz Clinical Trial Management System (CTMS) built on the Salesforce cloud platform is an integrated clinical trial operations management solution. It offers management of the entire processes with real-time visibility and analytics across study planning, budgeting, start up, study management, and close out.





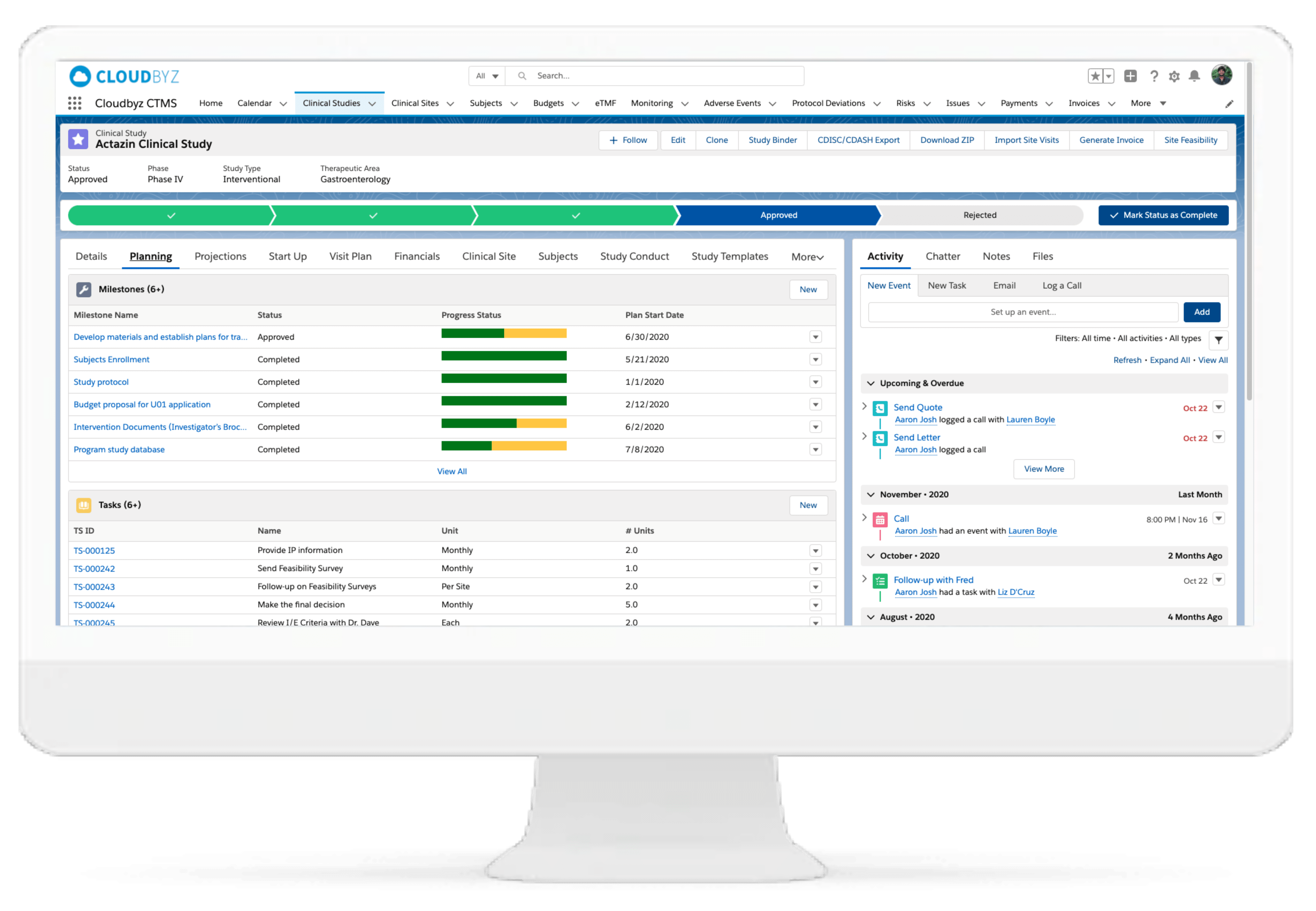
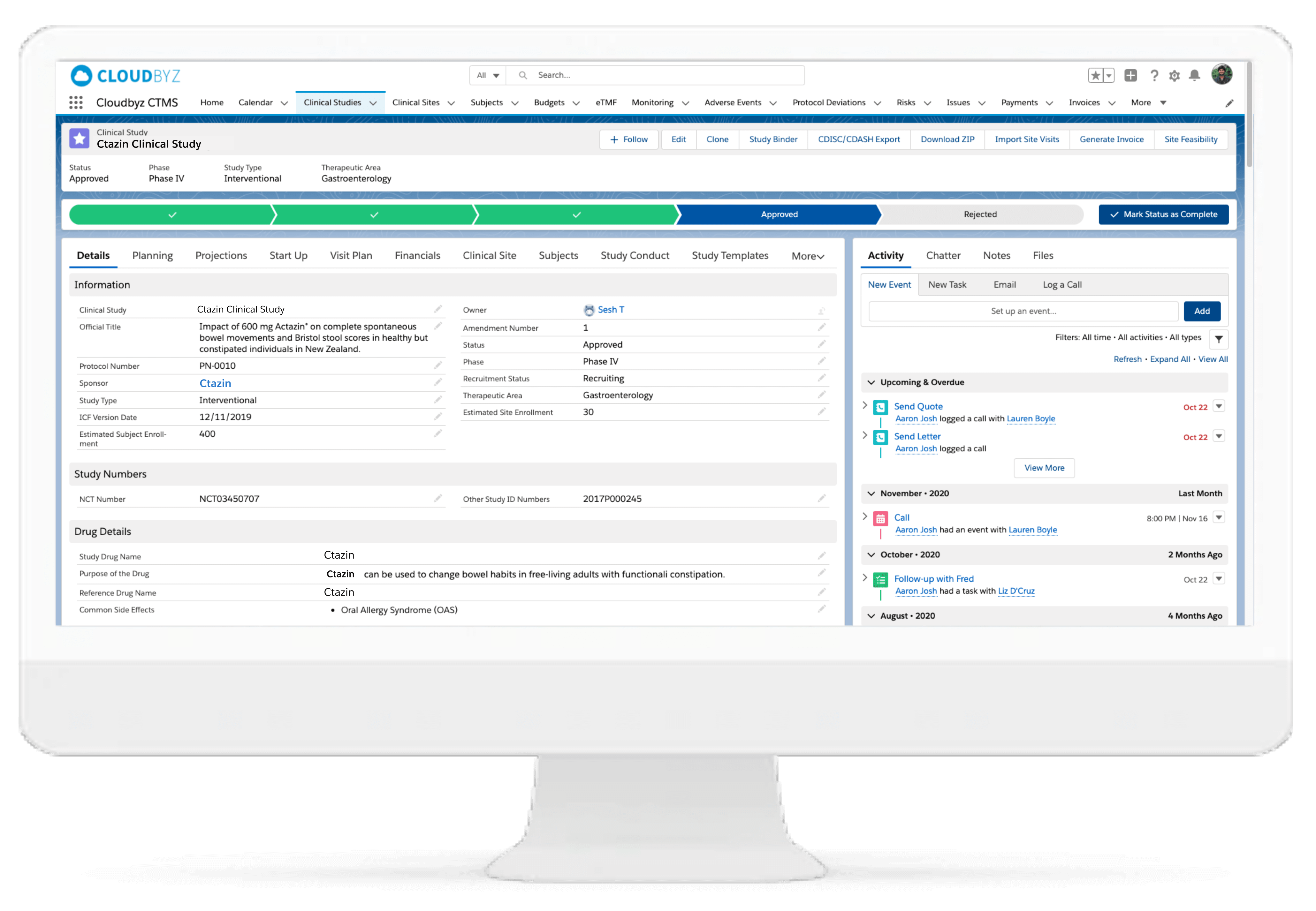
Cloudbyz CTMS solution offers end-to-end project management of the clinical trials. Set up and visualize studies according to protocol requirements and easily access details such as phase, type, therapeutic area, indication, outcomes, eligibility, etc. Set up and track protocols and amendments.
Track ethical and regulatory approvals and access IRB-approved content from one place. Organize the content by approval and expiry dates. Additional features include milestones and tasks management, study teams’ management, training management.





Manage and engage with sites directly from the system. Assign sites to a particular study and track their documentation, credentialing, equipment, facilities, and activation status. Deliver content and services to clinical research sites through the investigator portal. Provide documents to all the sites through the system to keep the sites informed and engaged.





Create the study feasibility templates within the system. Modify and customize the templates for each study and site. Send feasibility surveys and record responses in real-time within the system. Sites can use fillable forms for quick and easy completion.
Evaluate the responses with the help of various dashboards and reports. Save each site’s credentials for future reference and use. Generate and save various feasibility metrics.





Set up a monitoring schedule based on the monitoring plan and Schedule monitoring visits. Identify data discrepancies through 100% SDV or set algorithms to perform SDV on specific and relevant data items such as safety data, enrollment, primary or secondary objectives, etc. Easily set up edit checks within the system and risk-based monitoring workflows including Key Risk Indicators, triggers, and thresholds.
Complete the trip reports with a robust review and sign-off process. Create reminders and notifications based on your workflows. Track monitor and site performance with smart metrics and reports. Design and send the selection, non-selection, and activation letters directly from the system.





Cloudbyz eTMF solution offers a cloud-based repository of all your clinical trial documents including files, images, information, etc. Digitally capture, manage, share, and store all clinical trial-related documents with centralized overview. Manage essential trial documents, stay inspection ready, and enable real-time visibility for CROs, sponsors, monitors and other stakeholders in a clinical trial. Features include pre-built templates, ZIP file downloads to facilitate file submissions, folder structure access at clinical study record level, restricted/user permission configured file access, archiving, and more.





Cloudbyz Randomization & Trial Supply Management (RTSM) solution is completely customizable per the requirements of a clinical study. Our system uses an integrated IWRS (Interactive Web Response System) and effortlessly handles simple to complex randomization schemes with provision for stratifications and multi arm studies. The system has the ability to perform all the tasks related to trial supply management such as IP accountability, centralized inventory tracking, threshold management, automated orders and notifications.





Cloudbyz budget solutions enable a hawk-eye budget estimation, planning, payments management across clinical trials. Cloudbyz delivers defined budget templates, real-time tracking of investigator grants, procedures, and visit payments along with budget projections and variance between planned and realized expenses.
Set up and allocate study level and site level budgets for Start Up activities. Use templates to define budget for site specific costs and associated costs such as staffing, patient recruitment and retention, outsourcing, vendor selection and management. Generate metrics to compare spending and return on investment between various sites and studies.





Generate payments, create detailed invoices, view payment tracking history, and manage study and site level budgets with the Cloudbyz CTBM solution. Leverage template-based modules to set up multiple budget versions at the study and site level. Simplify accounting practices by managing and tracking advance payments to sites, partial payments, unscheduled and invoiceable items payments and holdback payments. Designed to fit your financial needs, set up different payment frequencies per site such as monthly or quarterly payments along with robust approval workflows to keep your business on track.





Cloudbyz Patient Recruitment solution offers complete end-to-end patient recruitment SaaS support across all types of clinical trials, catering to their respective requirements. The solution significantly empowers the sponsors, CROs, and sites to digitalize the entire recruitment process along with the smart features of automatic roll-ups, minimal hand holding, better metrics, and improved analytical capability.





Cloudbyz eConsent solution replaces the traditional paper-based consent documents with electronic files. This digitized feature delivers real-time document management of electronic patient consent with multi-device configurability. It includes template-based and interactive multimedia approaches to expand user understanding and information transparency.





Cloudbyz CTMS allows effective management of safety data for better risk management and response planning. It enables the clinical research team to monitor, track and report safety events. Timely tracking of protocol deviations enables the research team to deploy response planning including preventive action and corrective action.





Cloudbyz Investigator Portal solution provides a single platform to deliver content and services to clinical research sites. With the Investigator Portal, sites can complete eCRFs, report protocol deviations and adverse events, maintain electronic Investigator Site File, access study information, collect essential documentation, perform investigation product accountability, and communicate with monitors, CROs, Sponsors, and other vendors, all from a single location.





Select from the available reports and dashboards to view and assess critical metrics. Create custom and ad-hoc reports through just point and click configuration. Subscribe to your favorite or most important reports and receive them in your inbox at your chosen time and date. Powerful graphs and analytics present critical site and study metrics that significantly increase effectiveness for current and future trials.
Cloudbyz offers innovative, built-on Salesforce, cloud-based clinical trial solutions for the entire clinical trial lifecycle. Our integrated solution with a customer-centric approach supports CRO, sponsors, and sites in conducting clinical trials efficiently across all the phases.