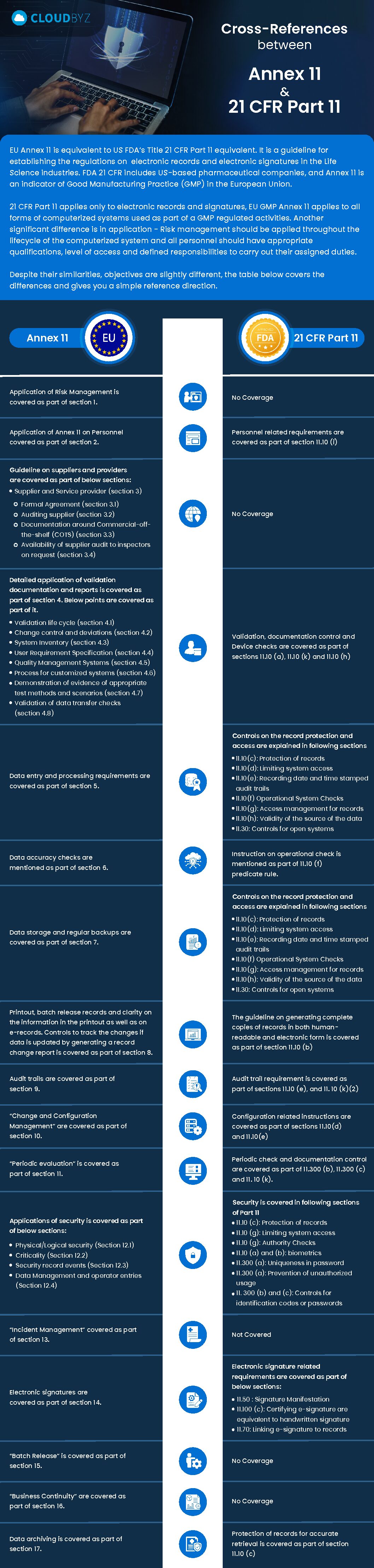
Regulatory Compliance Coverage & Reference between FDA 21 CFR Part 11 and EU Annex 11
Until recently, clinical trials have been performed in a mostly paper-based processes. Advancements in technology, surge in remote work and digitization of the processes is driving the clinical research industry…