Clinical Trial Data Management Best Practices
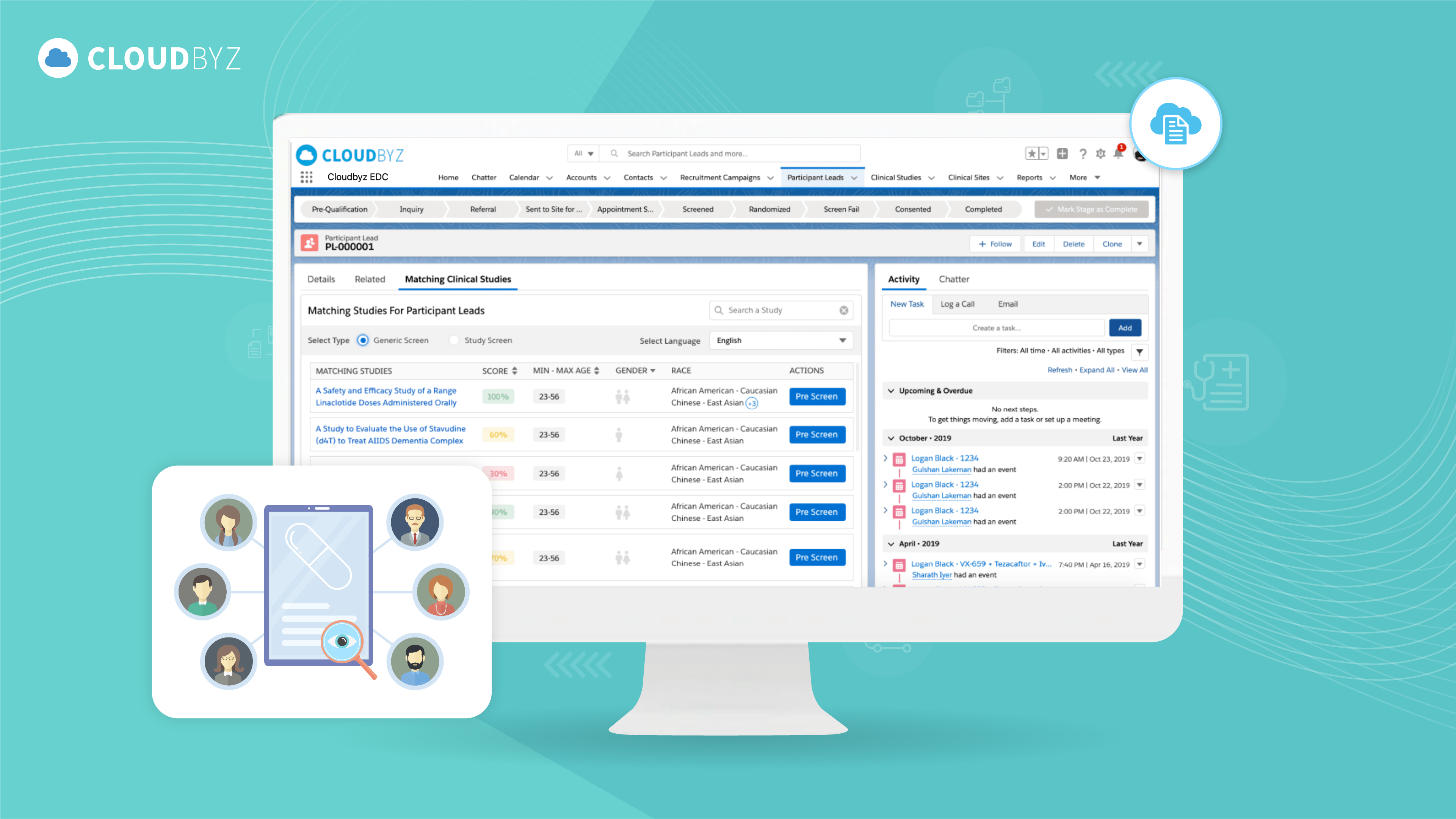
Clinical trial data management is a critical component of clinical research. Effective data management ensures the accuracy, completeness, and quality of the data, which is essential for regulatory approval and the generation of reliable study results. Best practices include the development of a comprehensive DMP, the use of standardized data collection tools, quality control processes, data security measures, regular backups, compliance with regulations and guidelines, performance monitoring, and documentation of all processes and procedures.