TRUSTED BY
Electronic Trial Master File
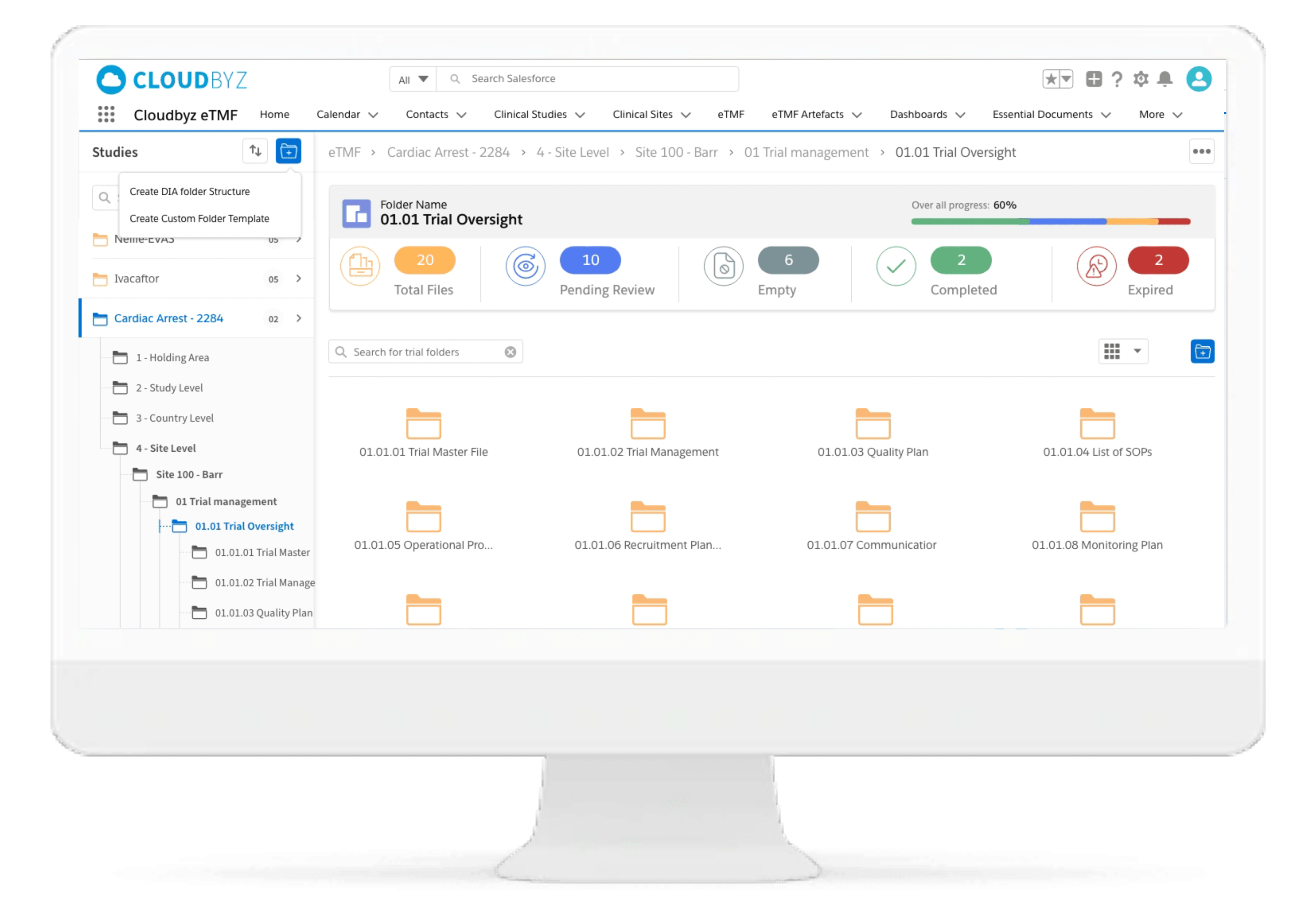
Cloudbyz eTMF solution offers a cloud-based repository of all your clinical trial documents including files, images, information, etc. Digitally capture, manage, share, and store all clinical trial-related documents with a centralized overview. Manage essential trial documents, stay inspection ready, and enable real-time visibility for CROs, sponsors, monitors, and other stakeholders in a clinical trial.
Product Features
Standard and Custom TMF reference Models
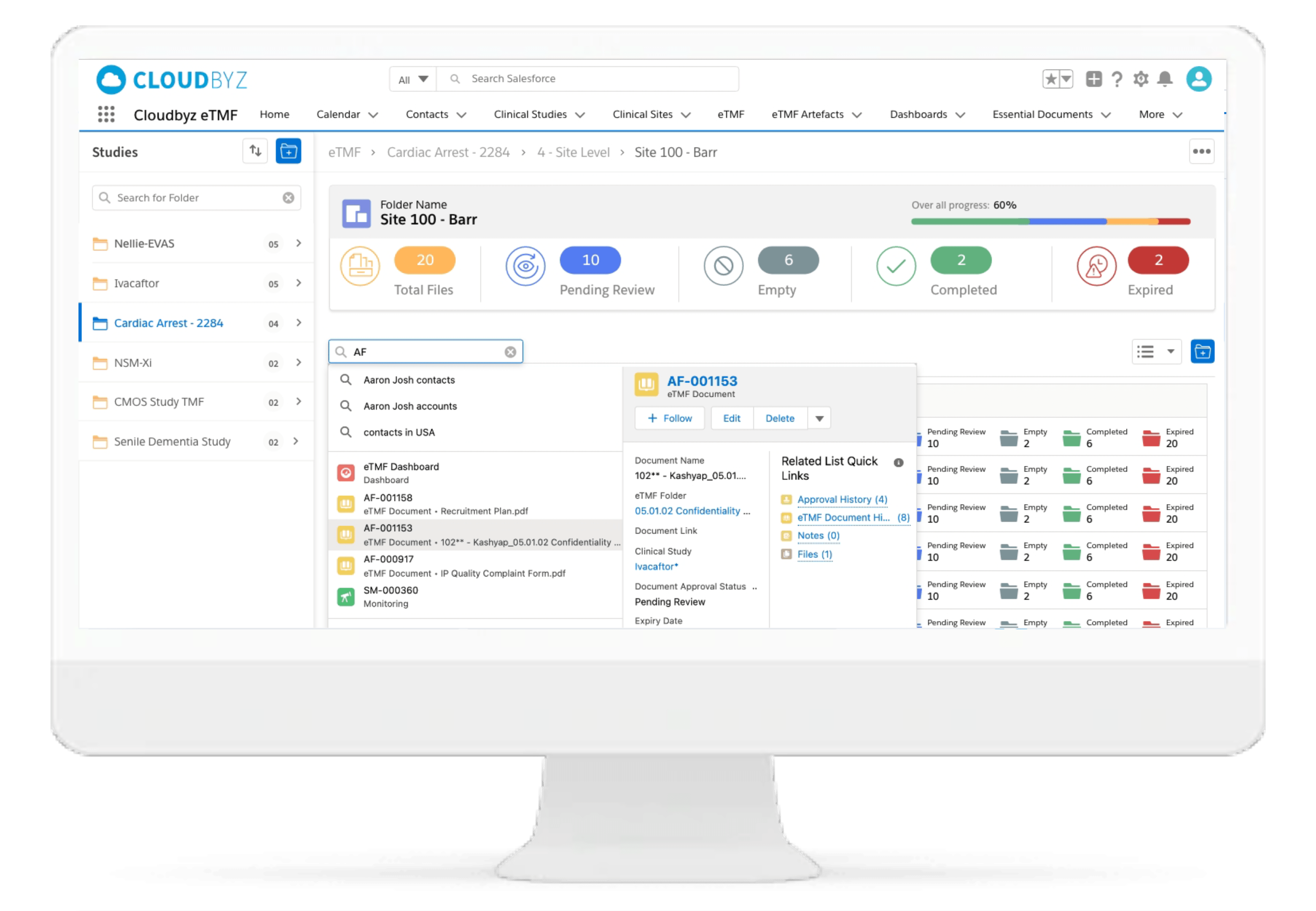
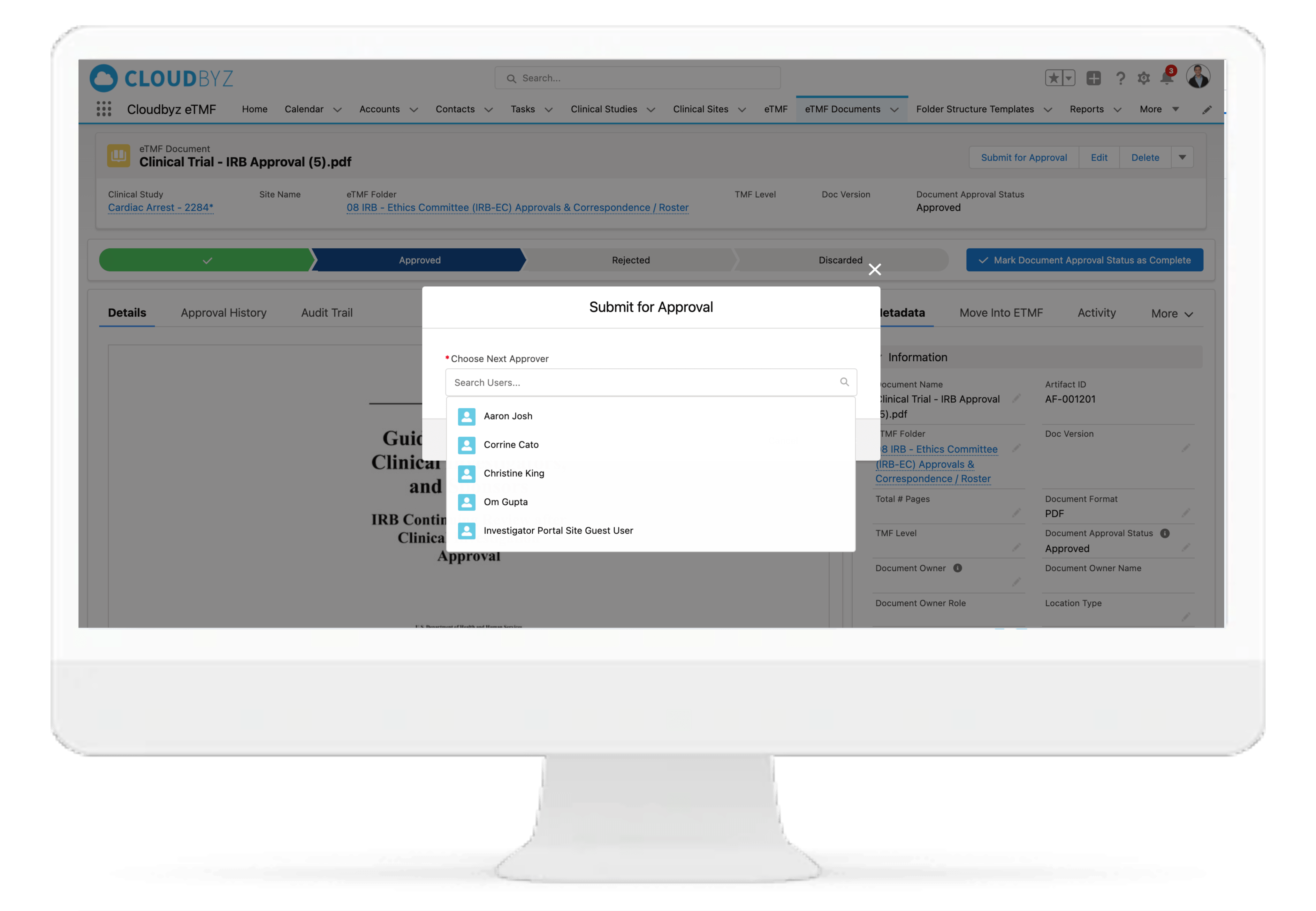
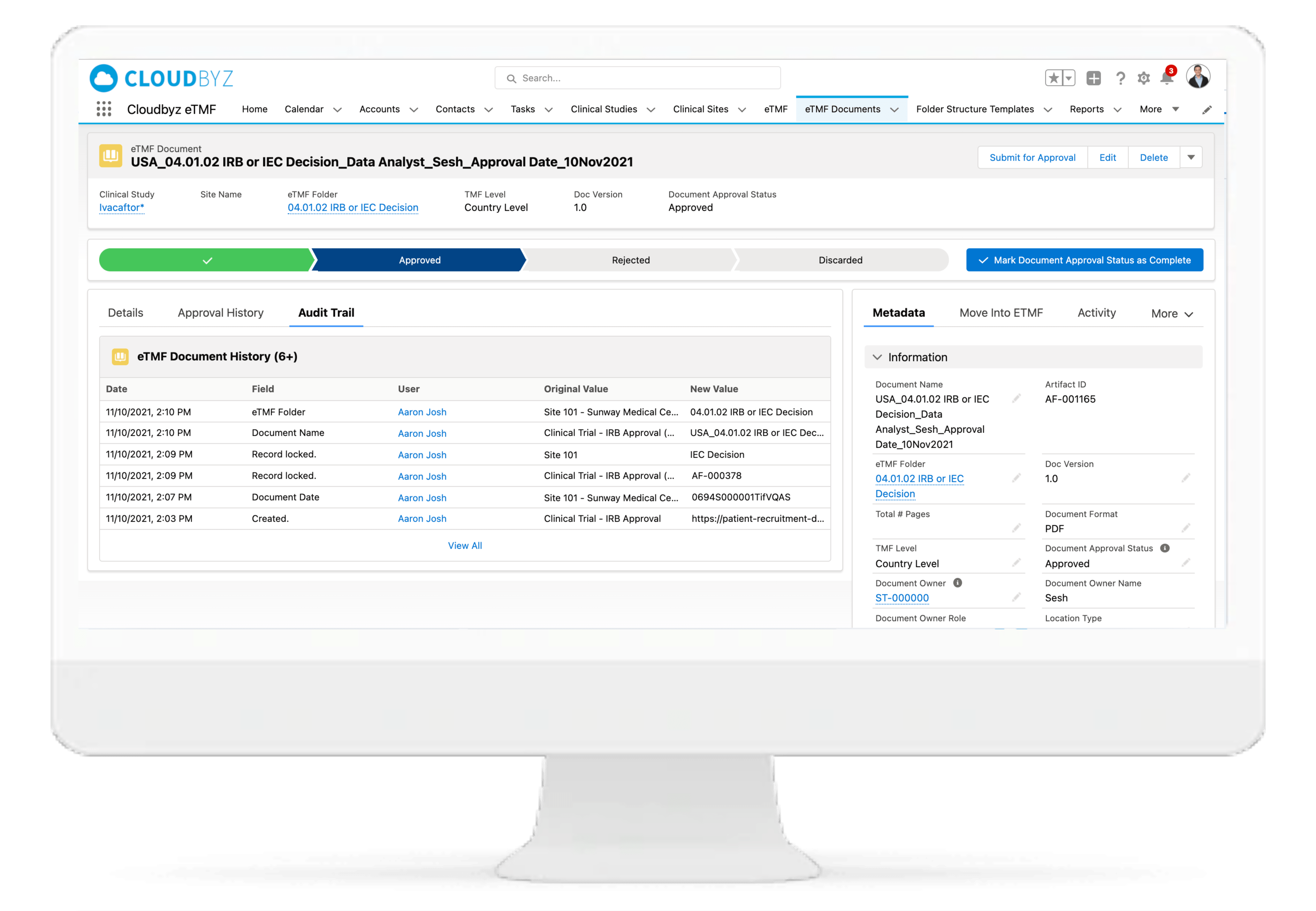
Review & QC Workflow
Review & QC Workflow
Built-in workflow routes documents through a review process with a click of a button. Assign document statuses such as "approved", "flagged", "discarded", etc. Send and receive notifications at each step of the review process. Customize the workflow based on the type and nature of a clinical research study.
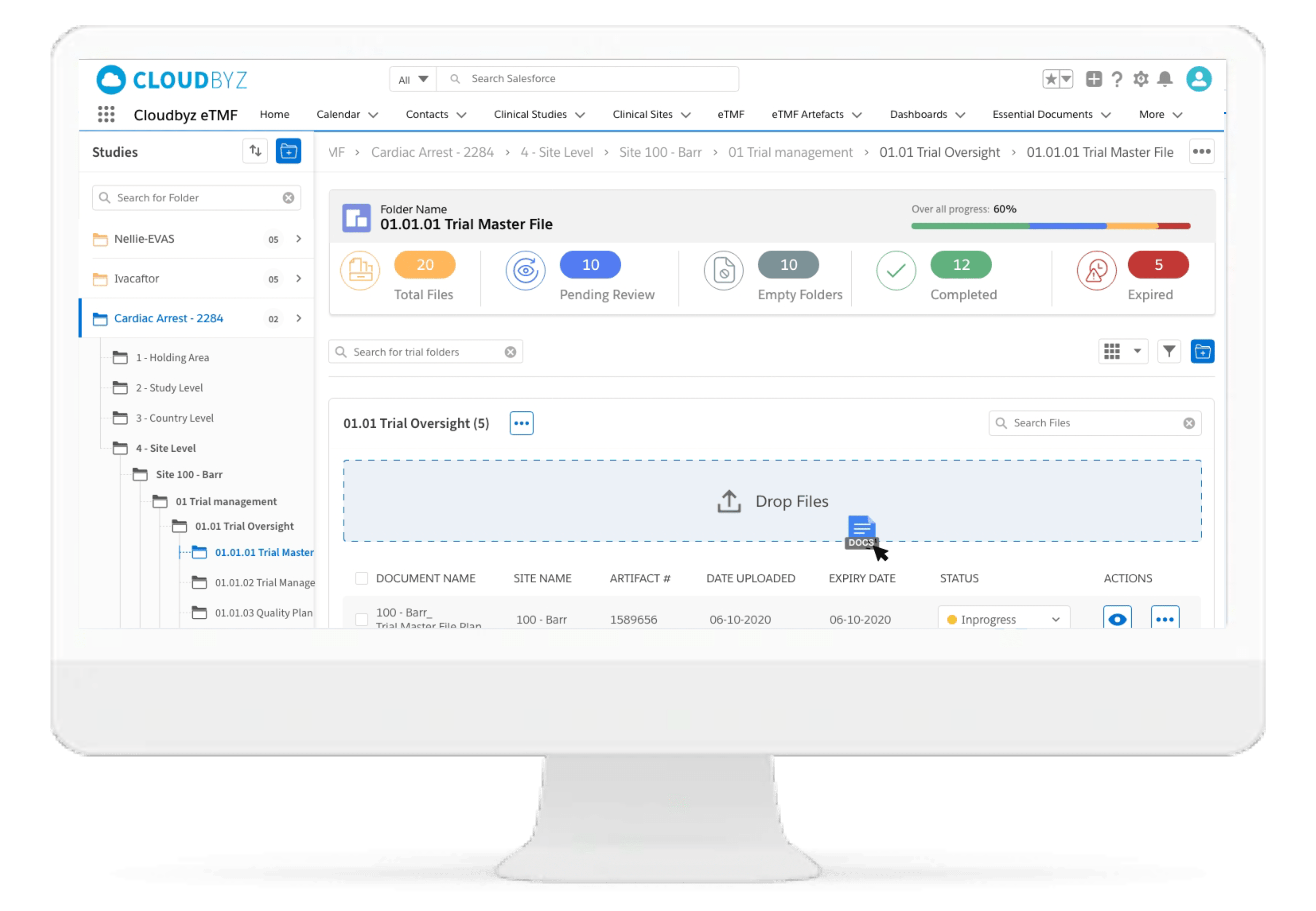
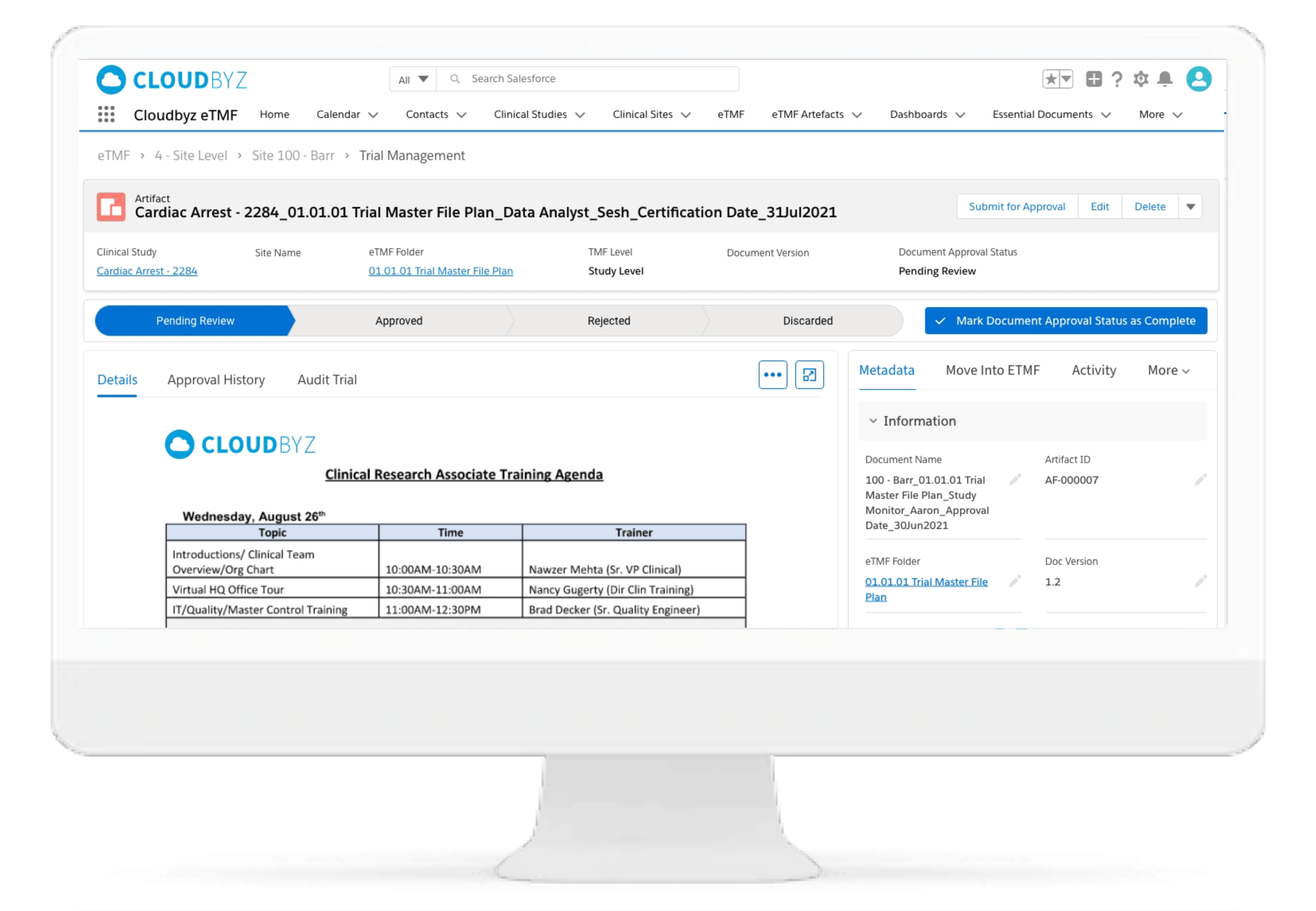
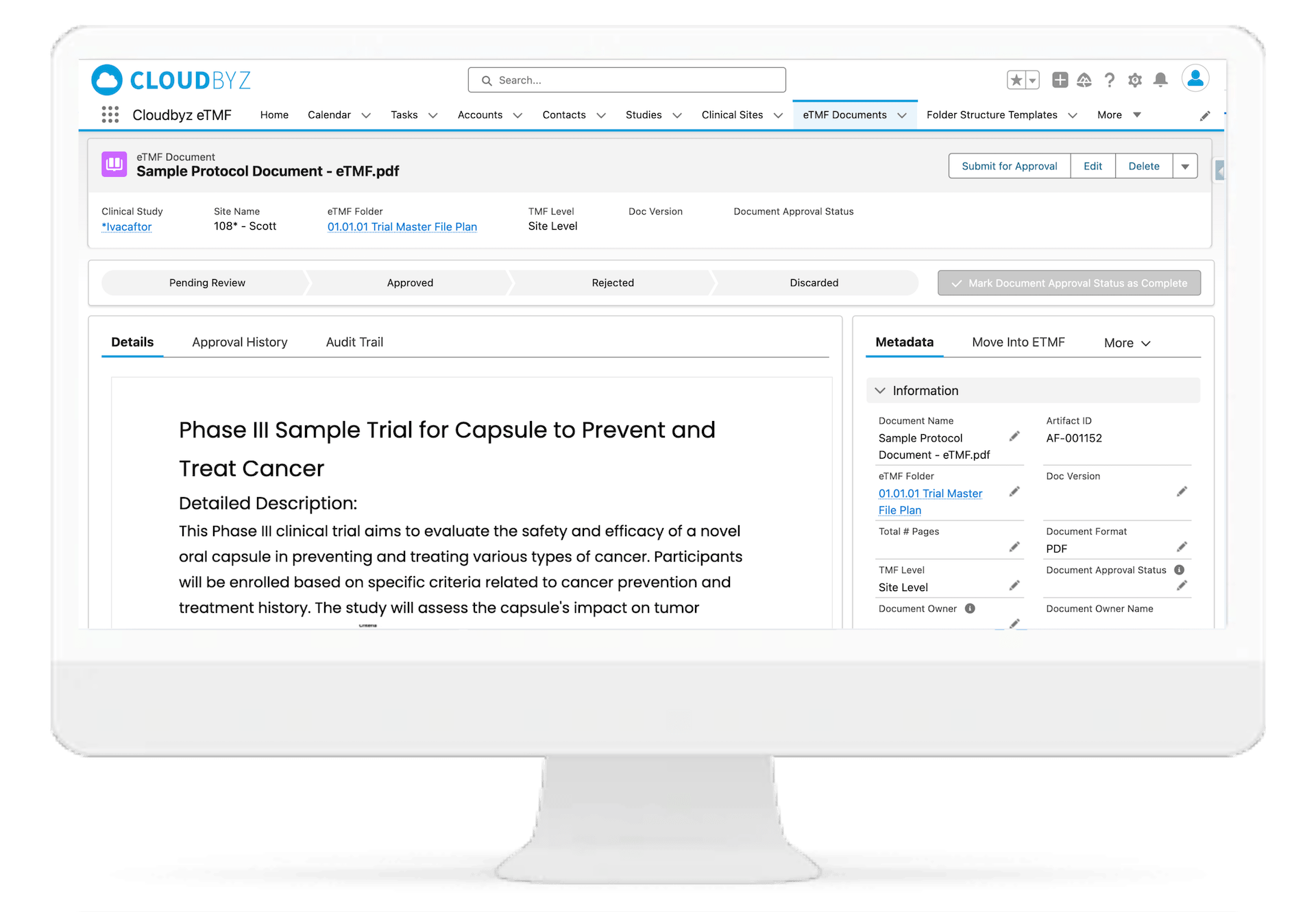
Automated and Configurable Metadata
Automated and Configurable Metadata
The solution enables research teams and stakeholders to see content and metadata side by side to eliminate misidentified documents and incorrect metadata. It empowers them to customize, define, and store an unlimited amount of metadata, as well as enabling them to access comprehensive metadata that offers auditors, inspectors, and users, a powerful and dynamic search capability for quick retrieval of documents. Auto-populate a study’s metadata to the maximum extent possible.
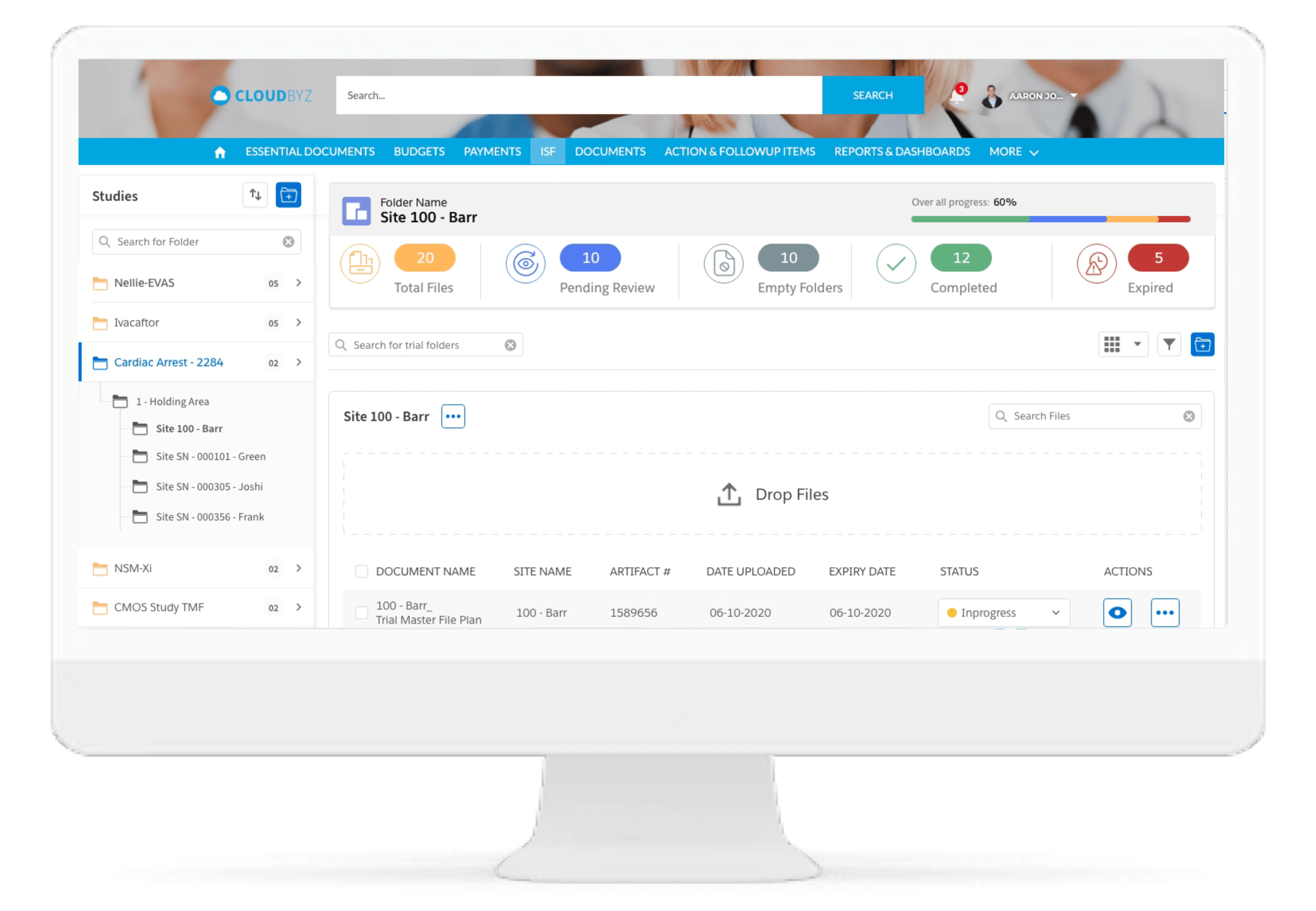
ISF Reconciliation
Chat, Email, and System Notifications
Chat, Email, and System Notifications
The solution provides timely, contextually relevant notifications to complete tasks, such as document reviews within the system, and cross-functional communication within the team using the chat functionalities. Set automatic Rule-Based Notifications to ensure timely actions on missing or expiring documents.
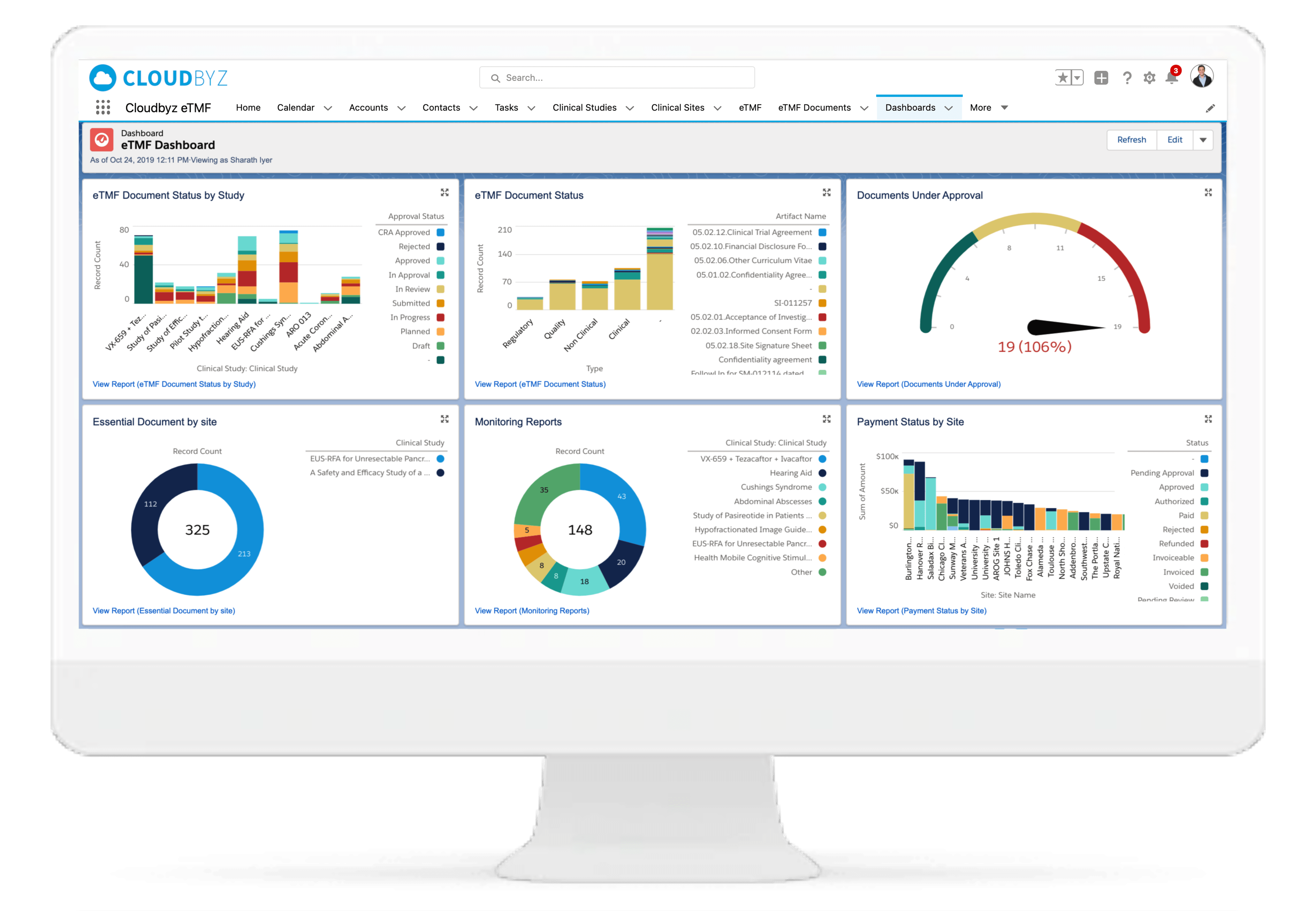
eTMF Reports, Dashboards, and More
eTMF Reports, Dashboards, and More
Use the eTMF reports and dashboards to view and assess critical metrics such as quality, timeliness, and completeness; in short, the pulse of their eTMF health. Establish workflows in advance and automate tracking, alerts, and reports if required. Subscribe to your favorite or most important reports and receive them in your inbox at your chosen time and date.