TRUSTED BY
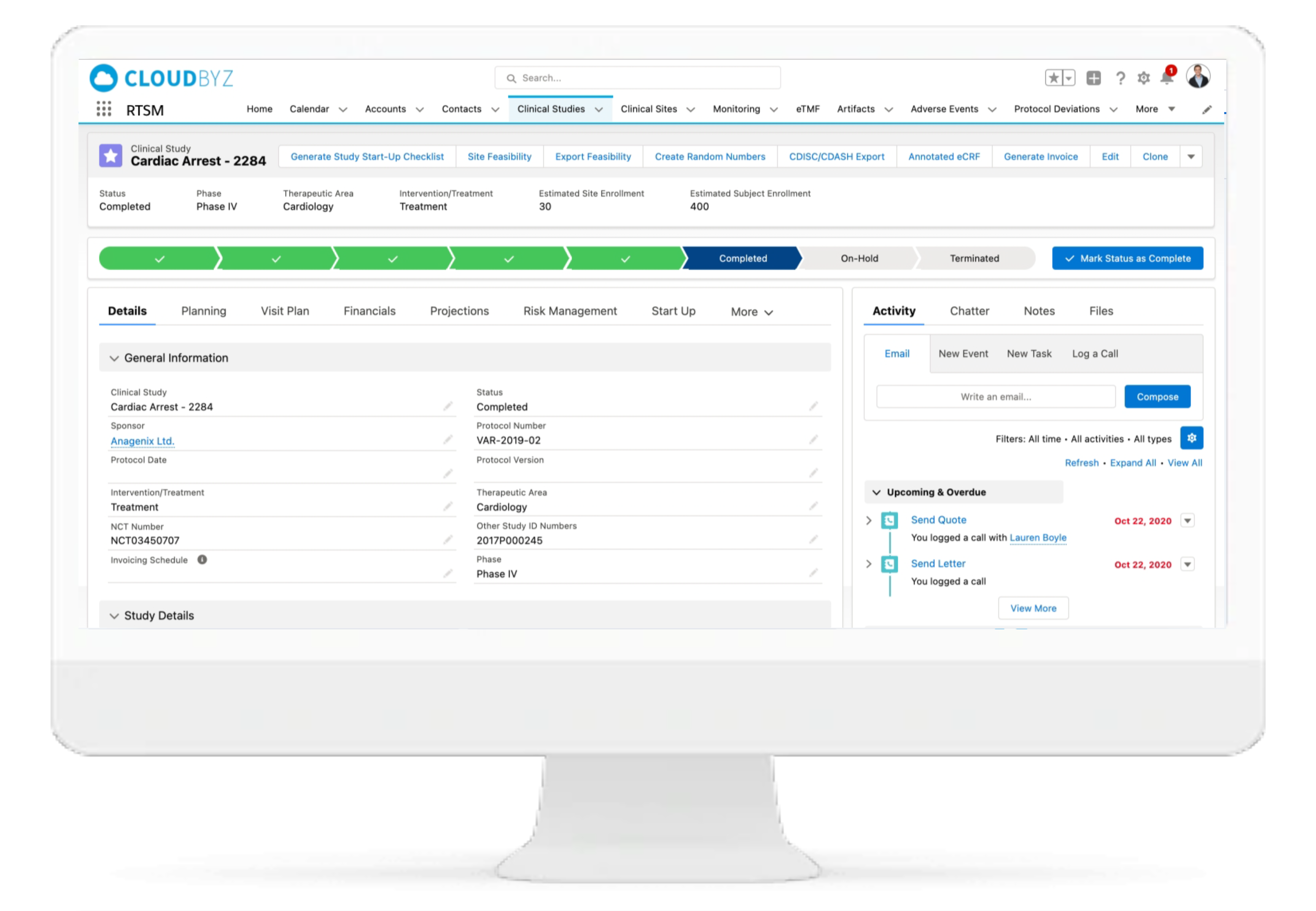
Randomization & Trial Supply Management
Cloudbyz Randomization & Trial Supply Management (RTSM) solution is completely customizable per the requirements of a clinical study. Our system uses an integrated IWRS (Interactive Web Response System) and effortlessly handles simple to complex randomization schemes with provision for stratifications and multi arm studies. The system performs all the tasks related to trial supply management such as Investigational Product Accountability, centralized inventory tracking, threshold management, automated orders, and notifications.
Product Features
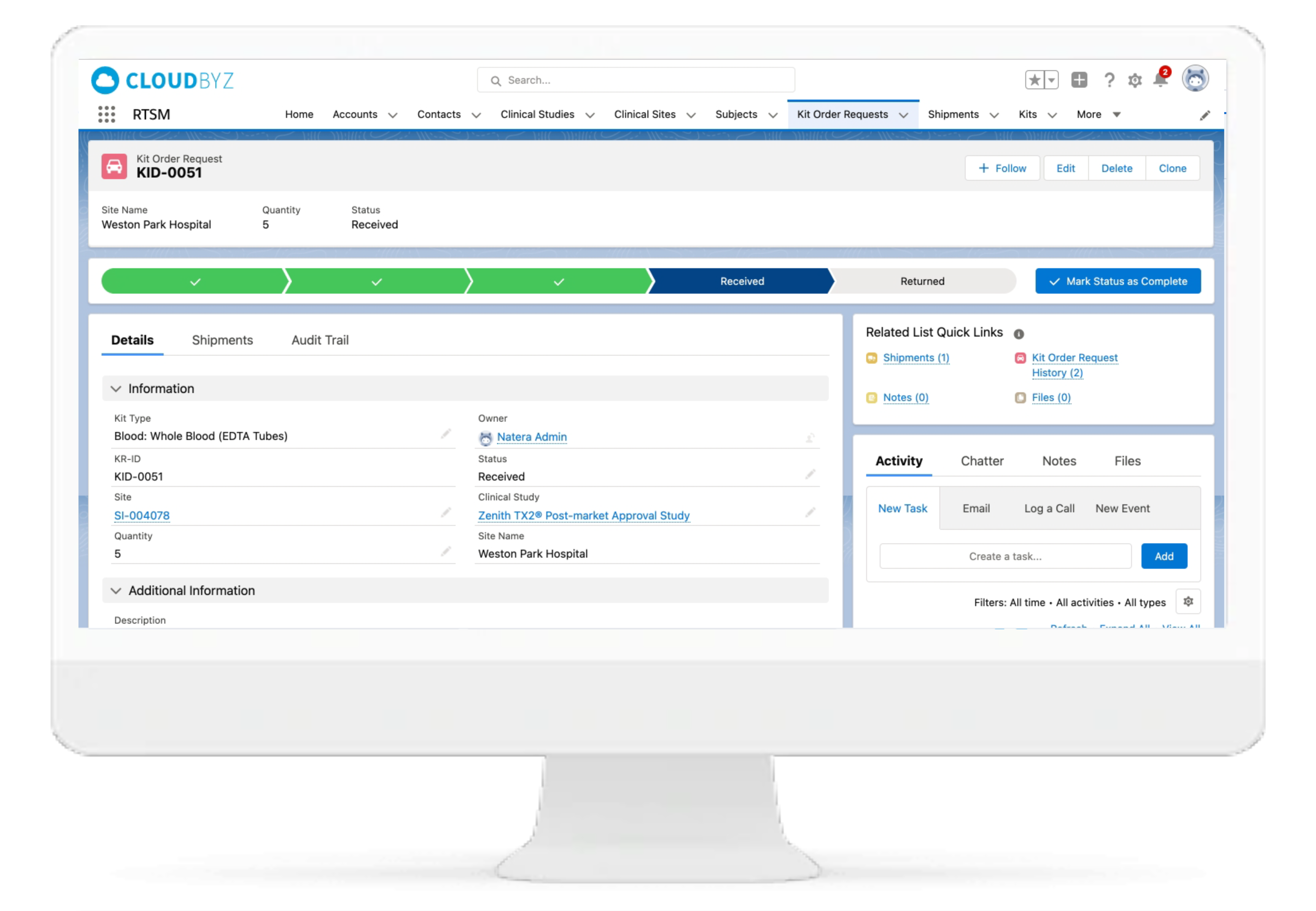
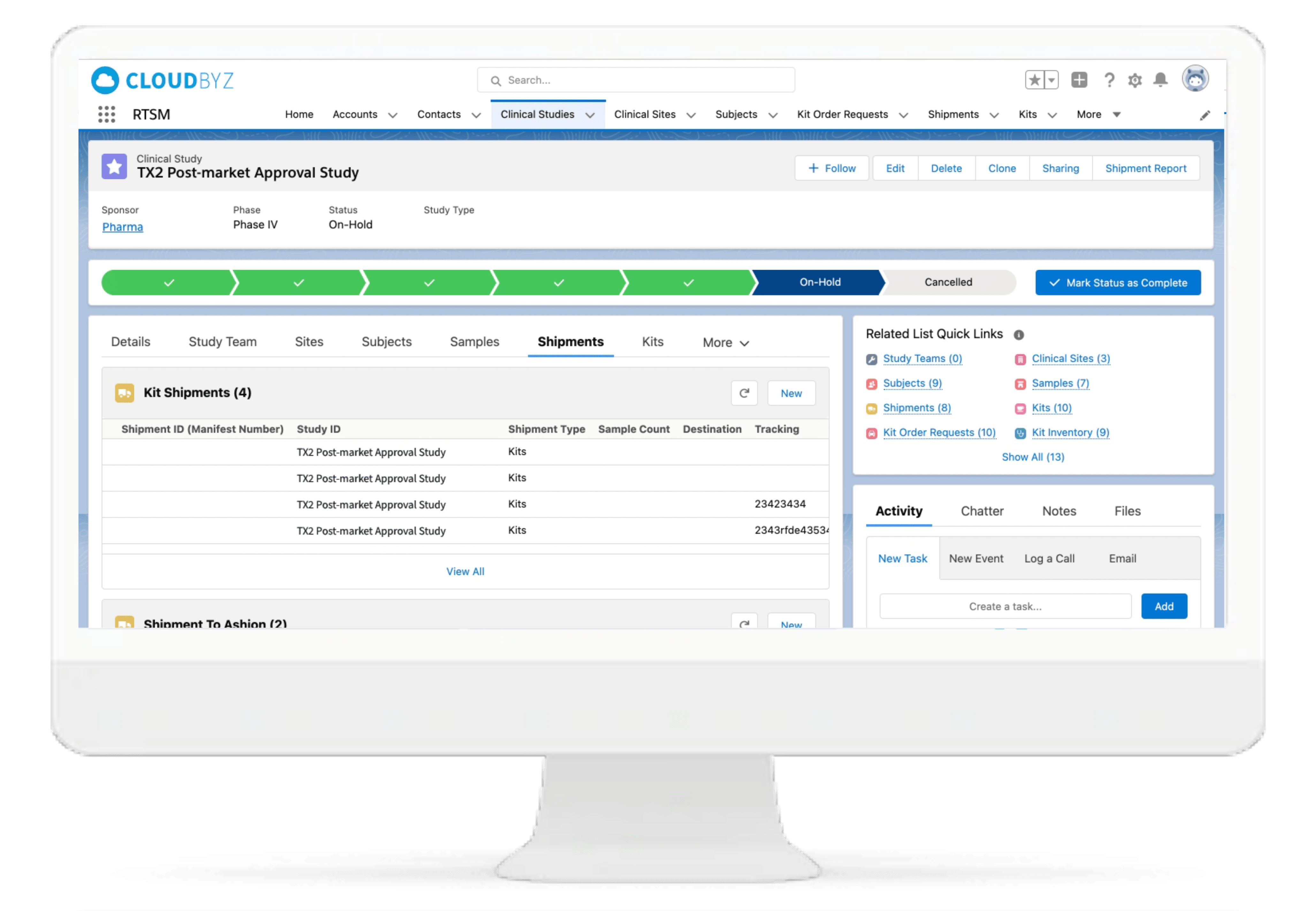
IP Orders Management
IP Orders Management
The Cloudbyz RTSM system tracks inventory at the site and confirms if a treatment inventory has fallen to a predefined minimum level. System sends an electronic request to the depot for consignment of additional supply to site. The orders can also be placed manually per study team's requirements.
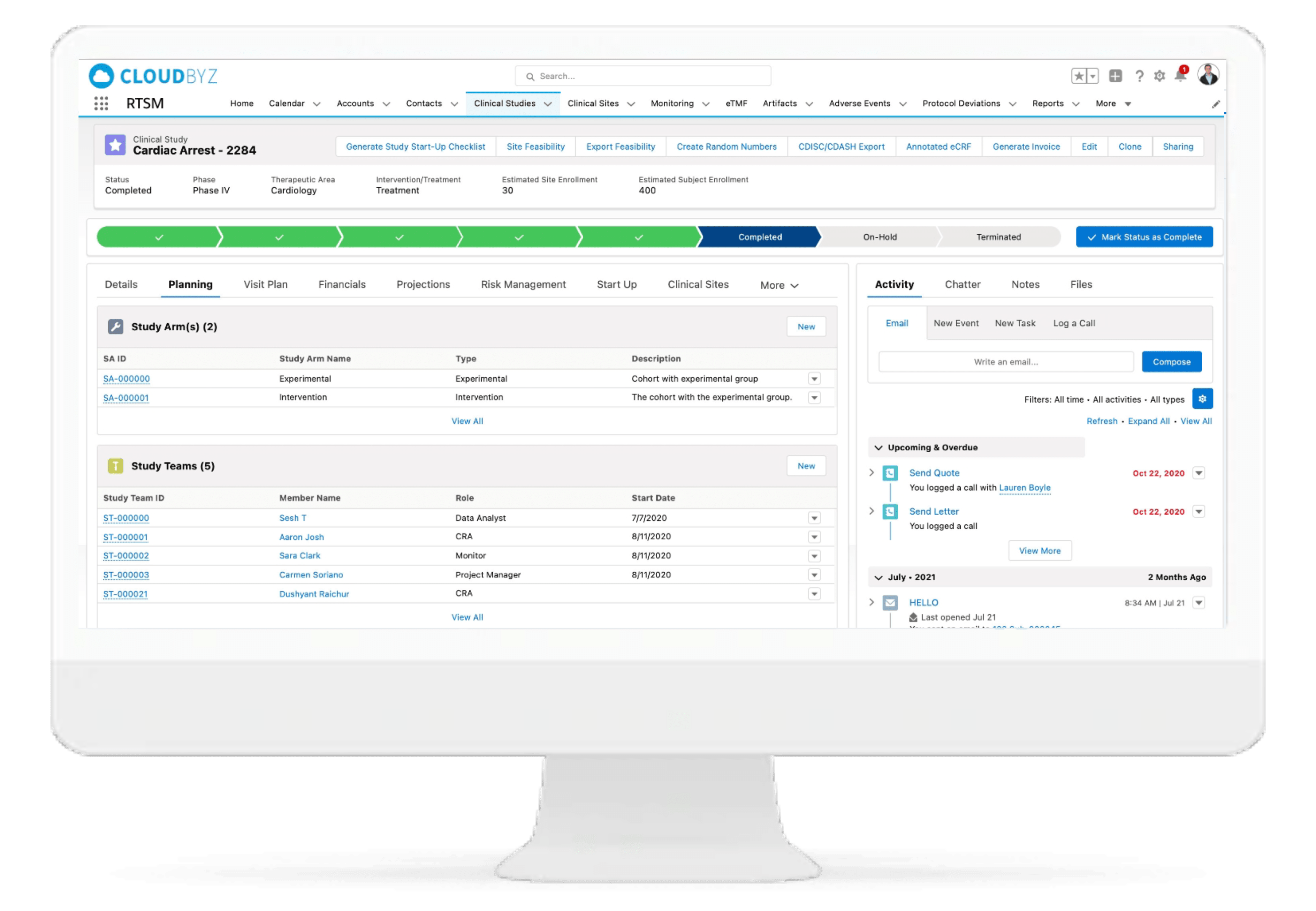
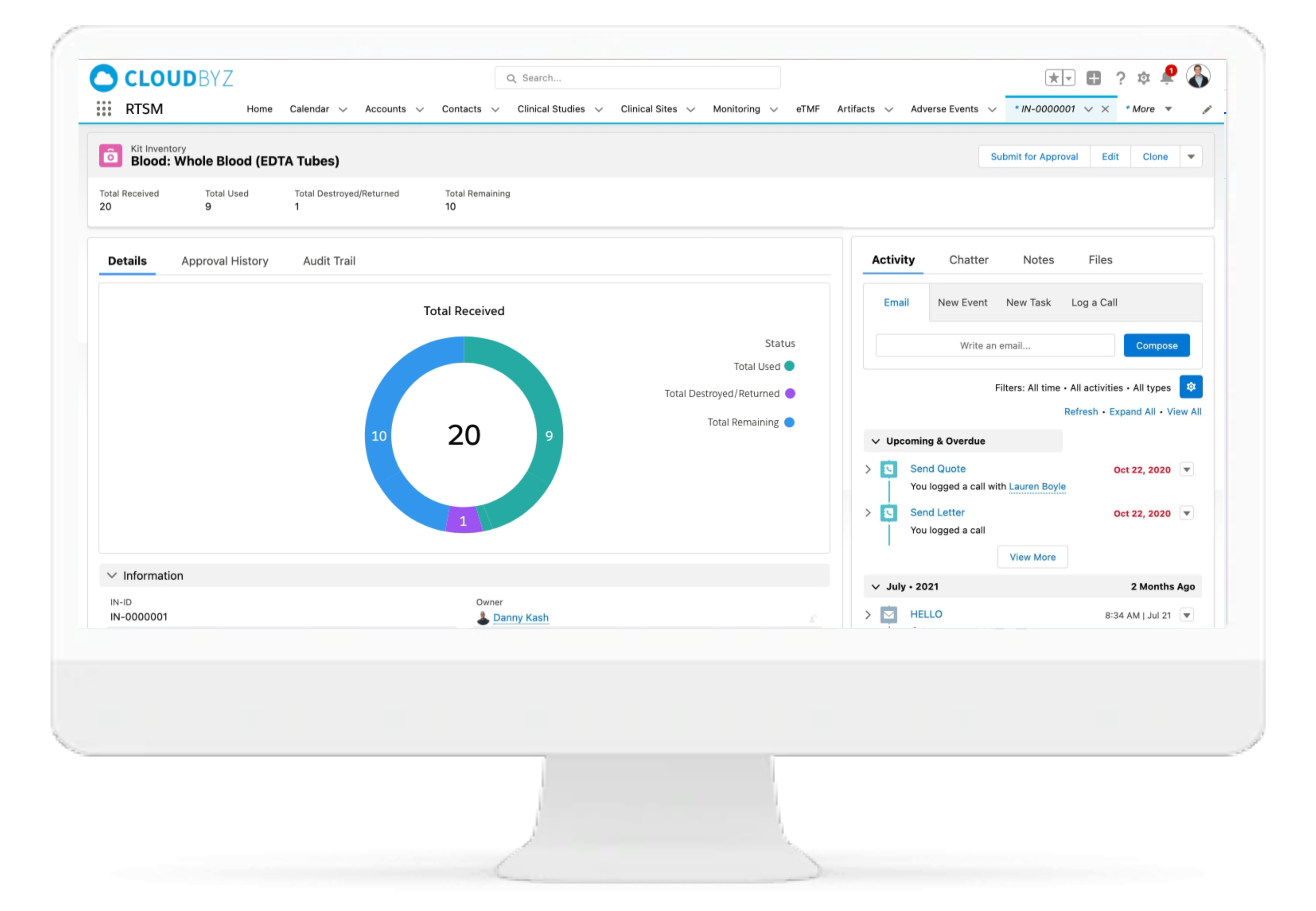
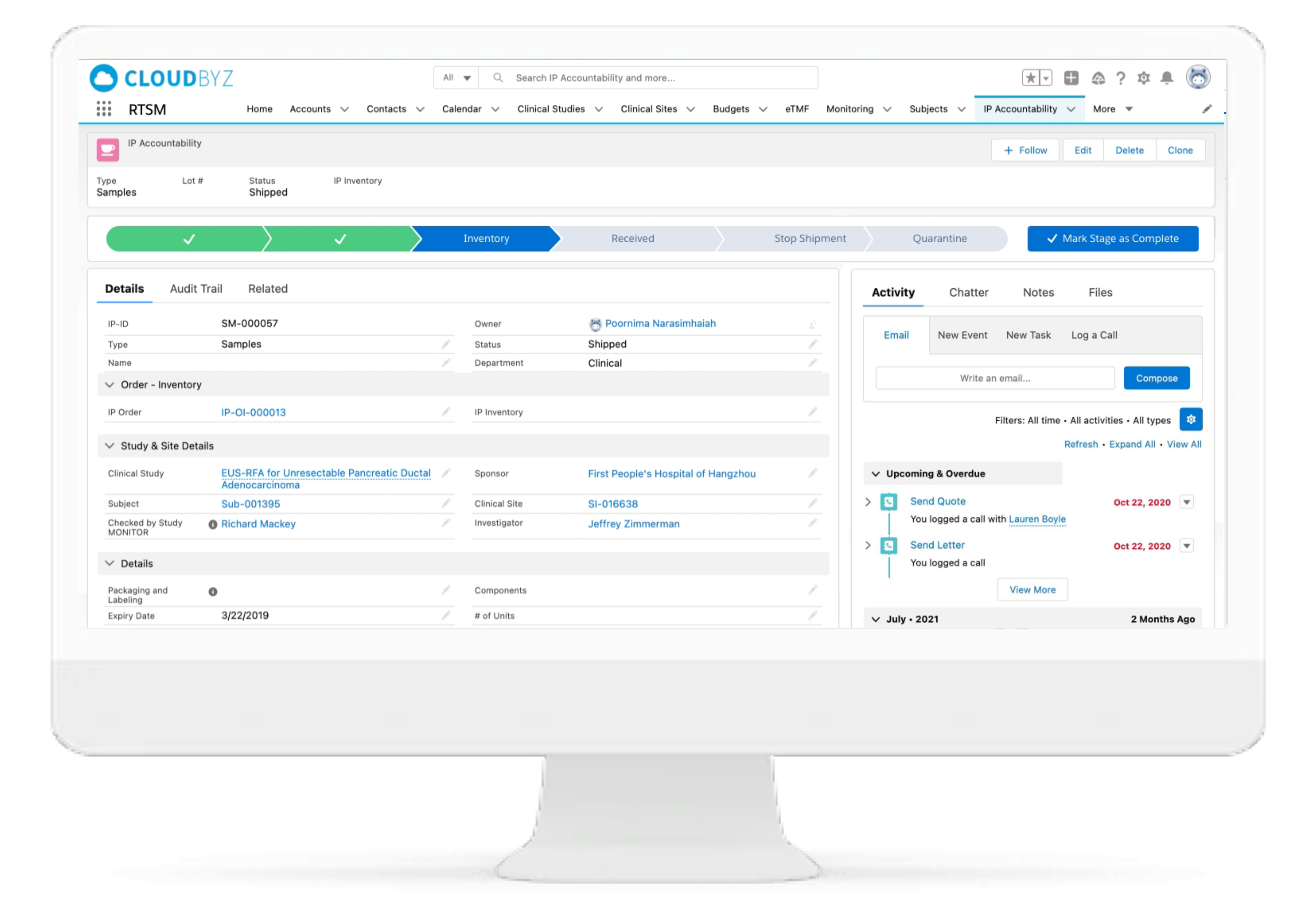
IP Accountability & Reconciliation
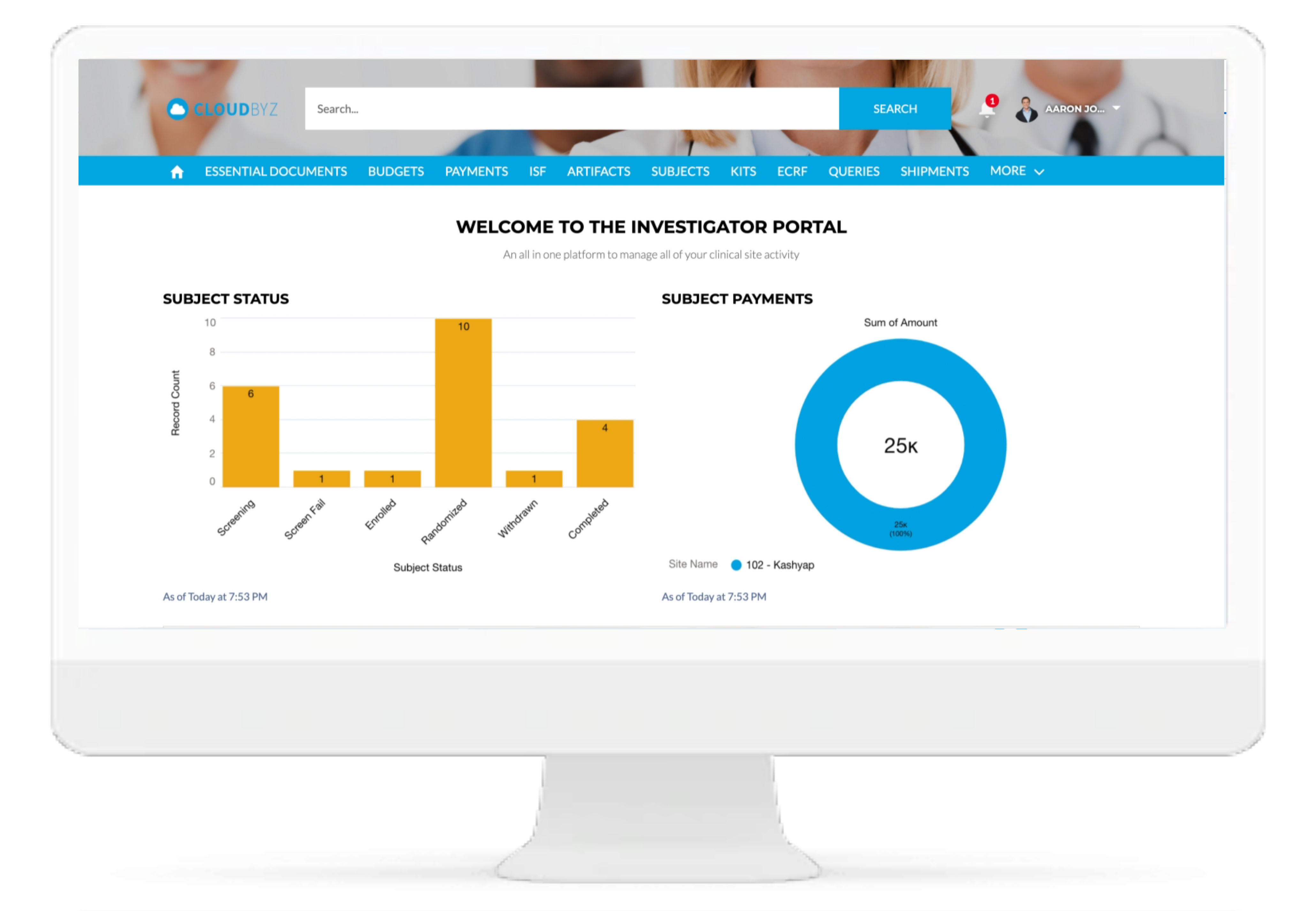
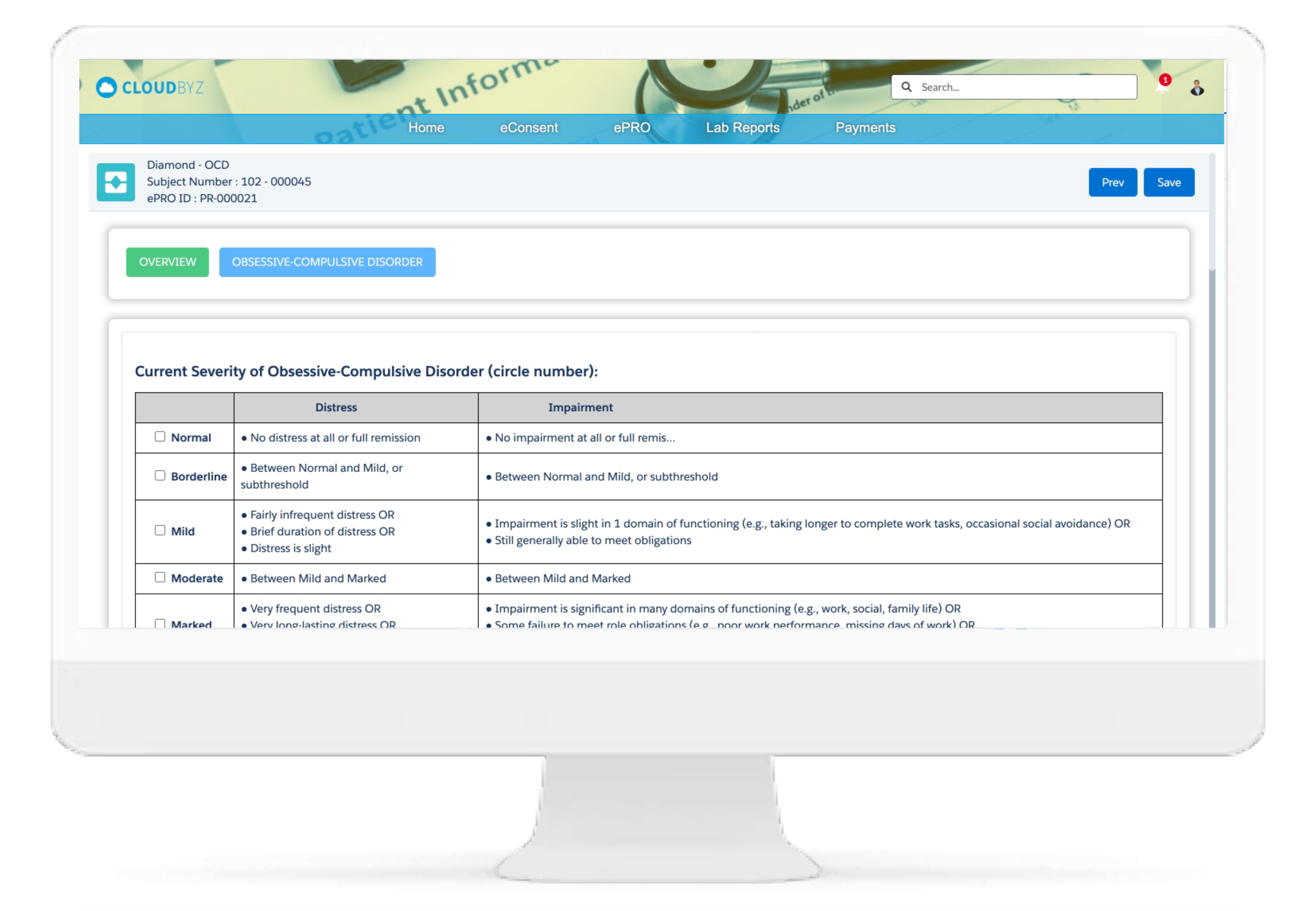
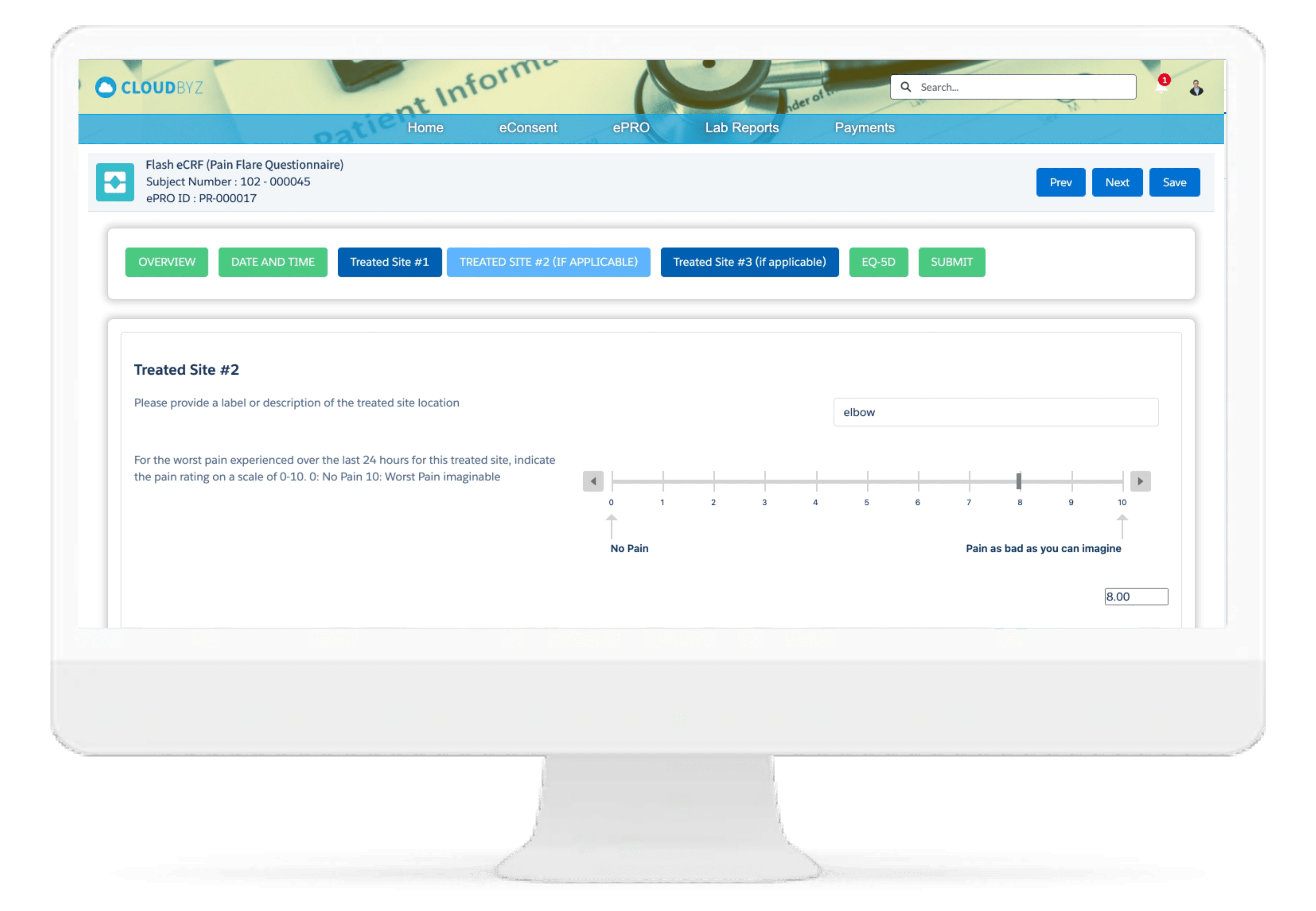
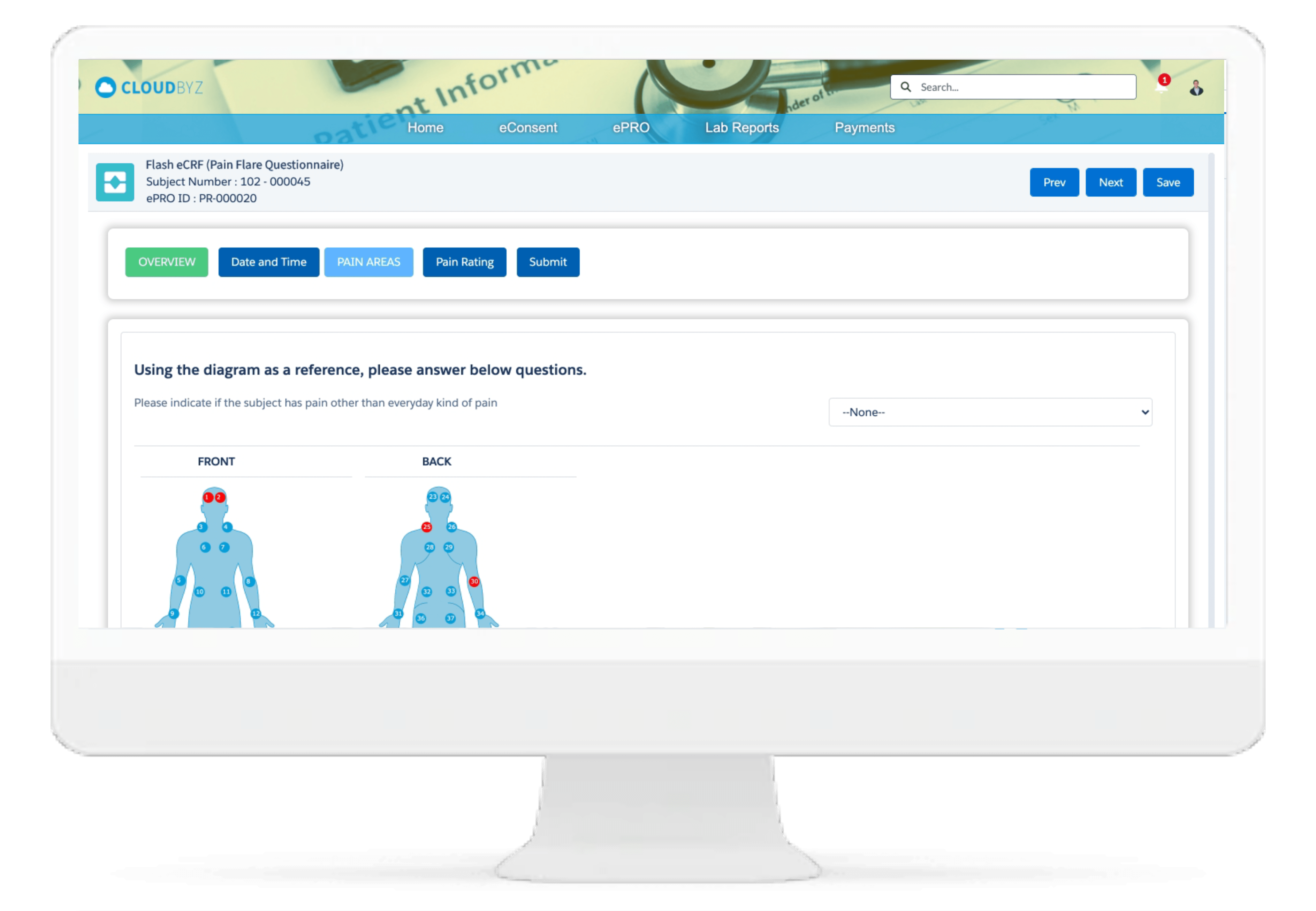
Investigator Portal
Investigator Portal
Cloudbyz Investigator Portal solution provides a single platform to deliver content and services to clinical research sites. With investigator portal, sites can complete eCRFs, report protocol deviations and adverse events, maintain electronic Investigator Site File, access study information, collect essential documentation, perform investigation product accountability, and communicate with monitors, CROs, Sponsors and other vendors, all from single location.
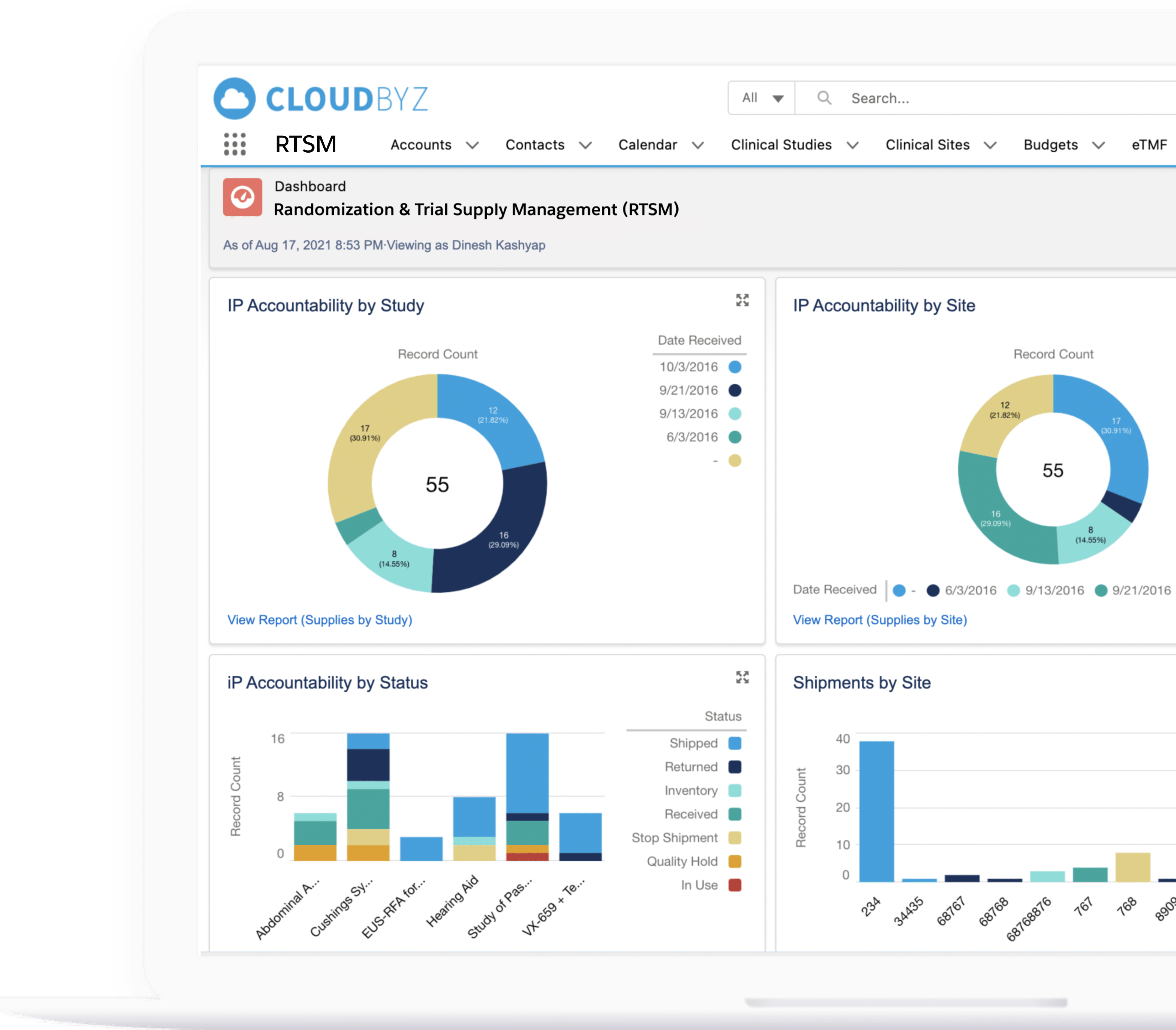
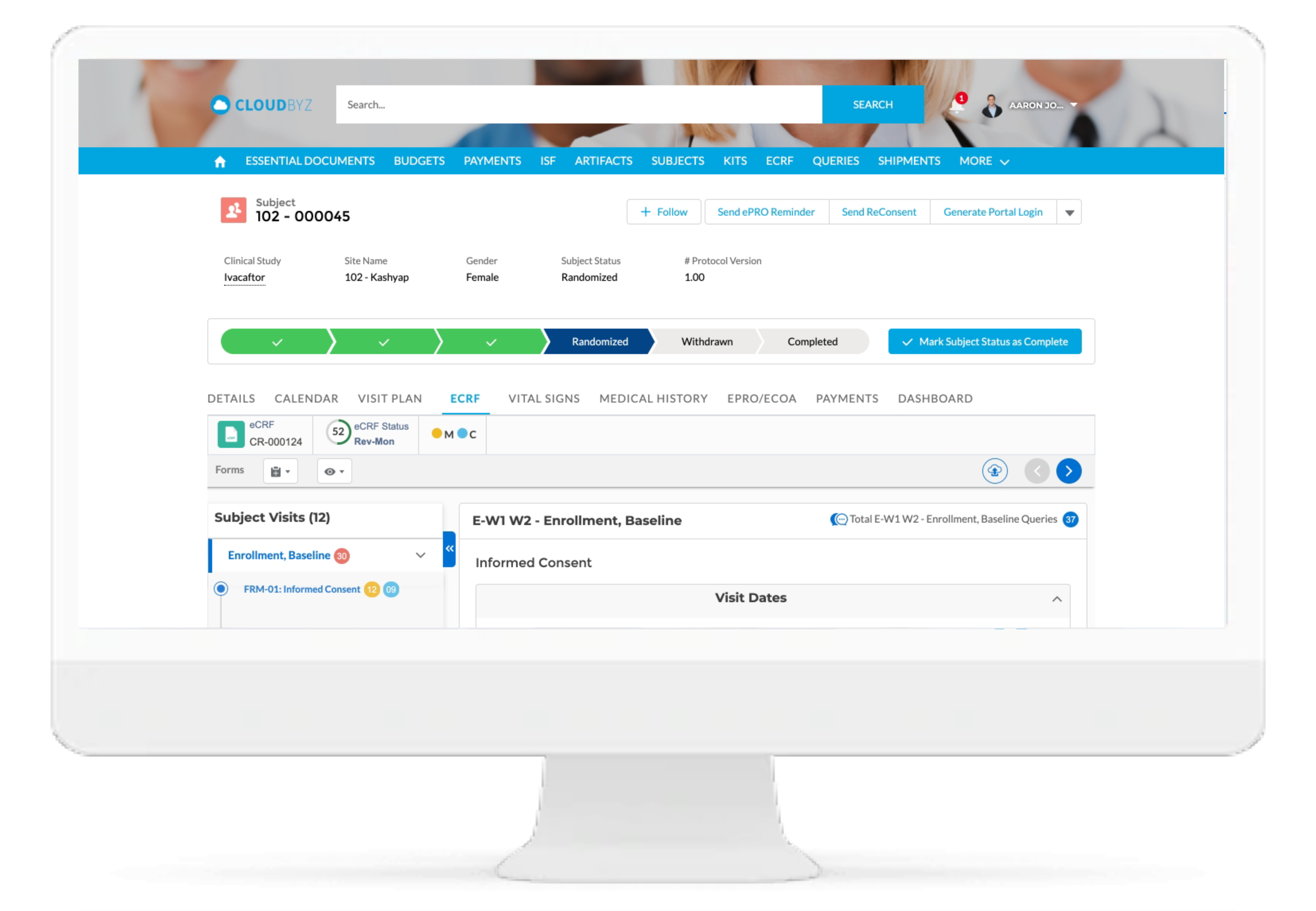
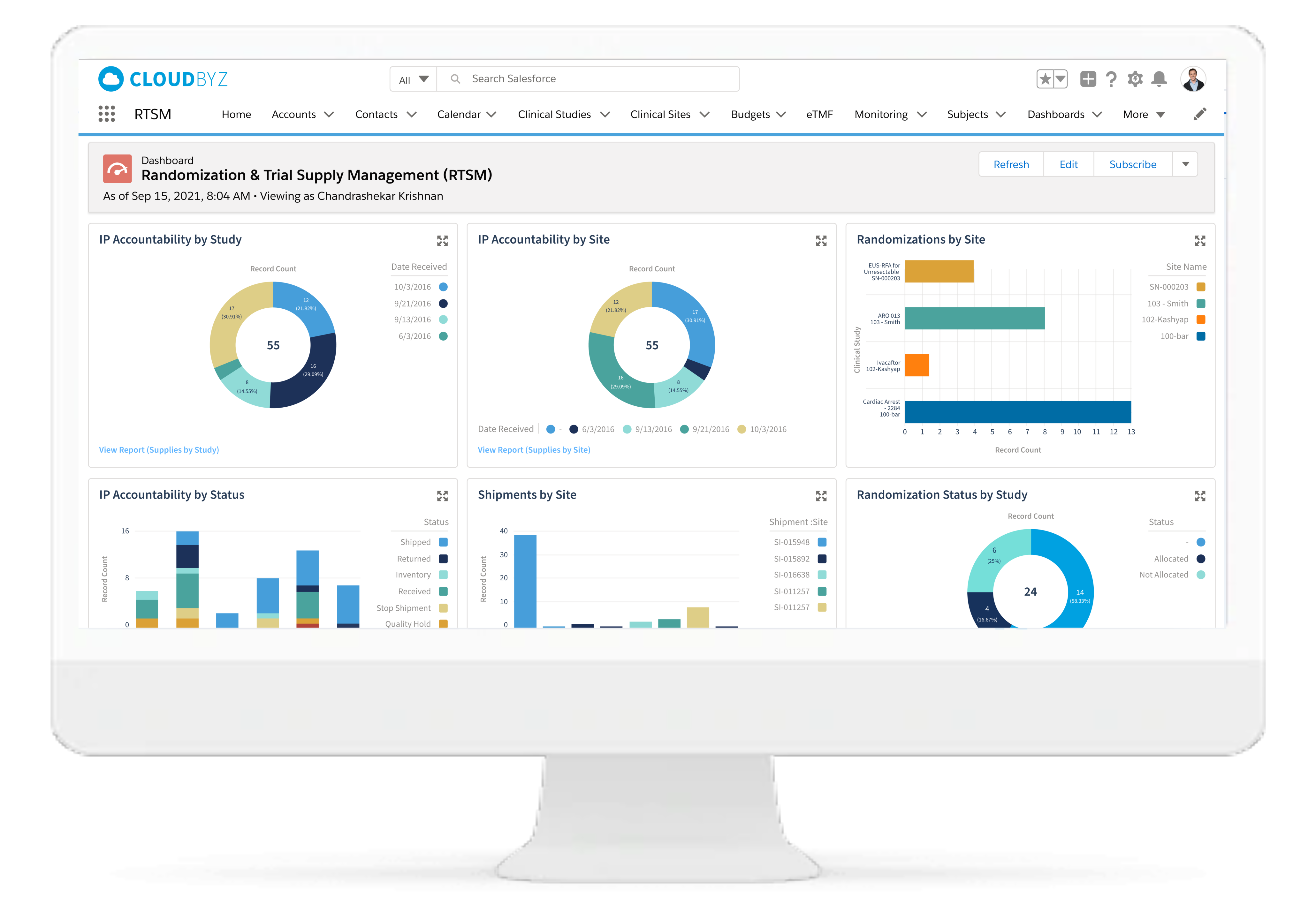
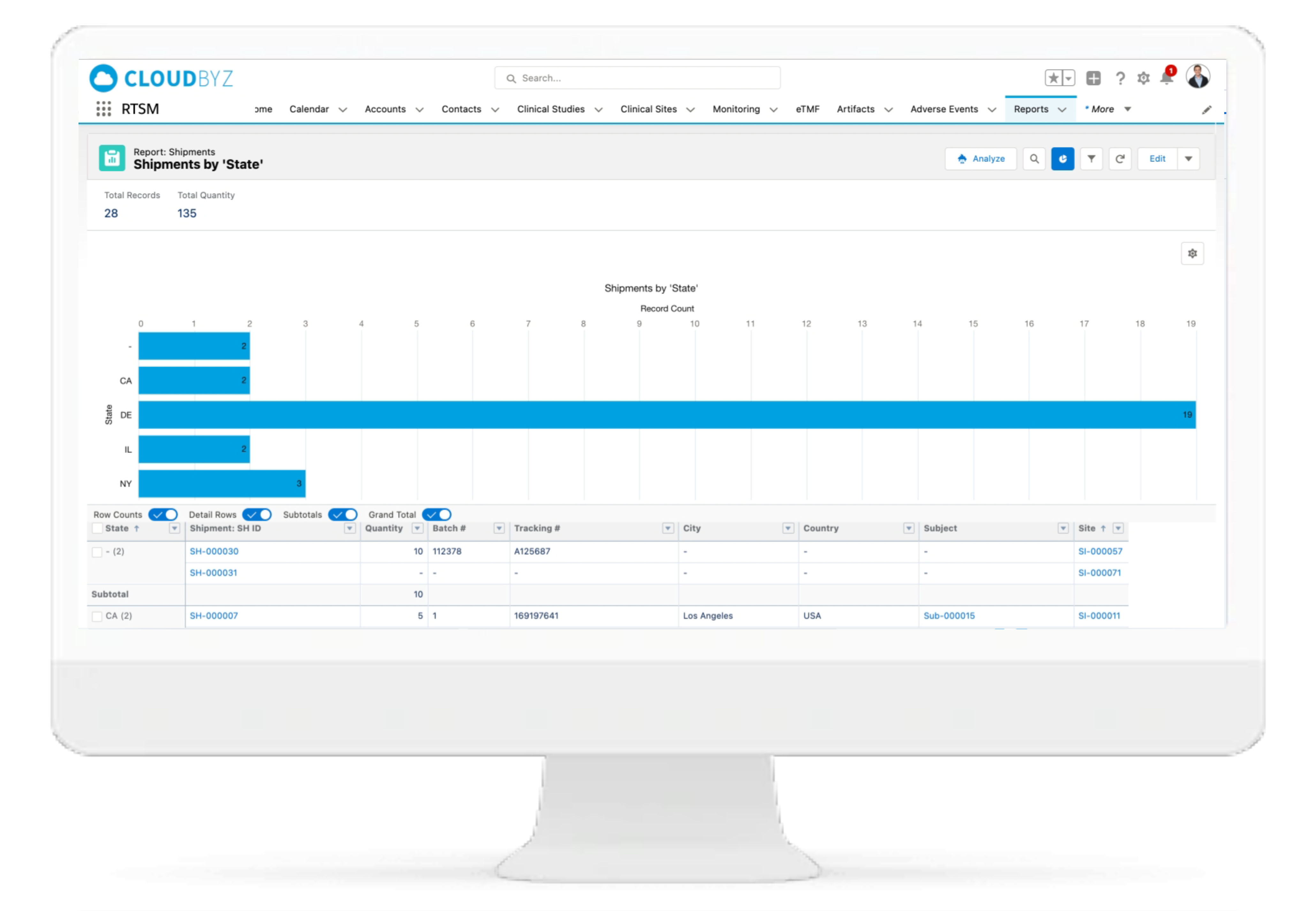
Real-time Analytics with reports and dashboards
Real-time Analytics with reports and dashboards
The Cloudbyz RTSM solution offers ready to use and customized reports and dashboards to track various metrics. Reports can be created with a point and click configuration to track metrics such as enrollment numbers, Inventory levels, shipment timelines etc.