
TRUSTED BY
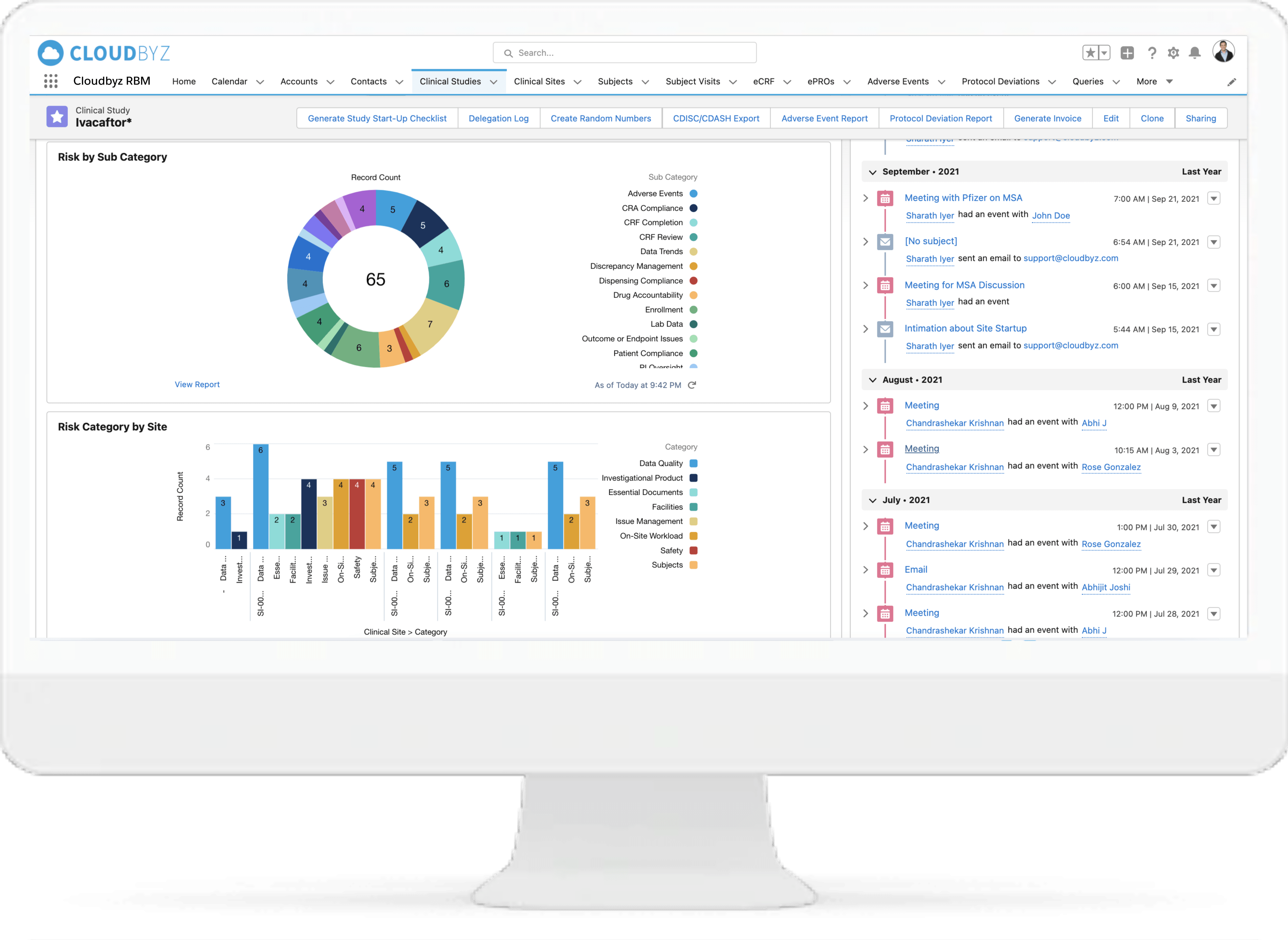
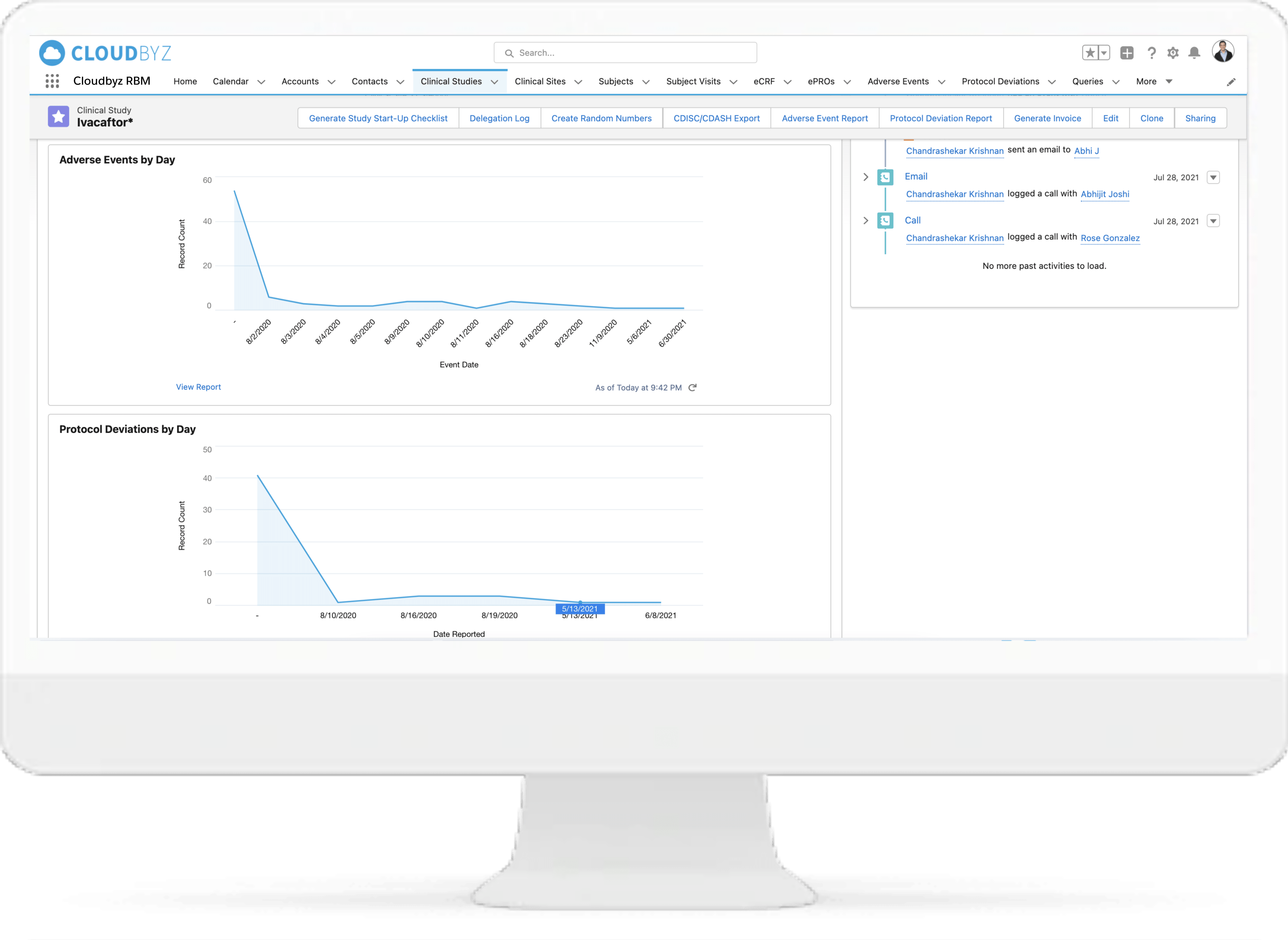
Cloudbyz delivers a Risk Based Monitoring (RBM) Solution that enables sponsors to improve their clinical oversight process, reduce time, costs, effort, and empowers them to review important monitoring activities, in real-time across the sites, all from one place. Our solution has the tools which offer quick results for the sponsors without the need for extensive programming, or even statistical experience.










Identify and apply the most critical data and processes for each type of study. Choose from a collection of Risk Indicators and apply to your study or set up your own. Manage key risk indicators directly within the system to increase data quality for the entire duration of the study




