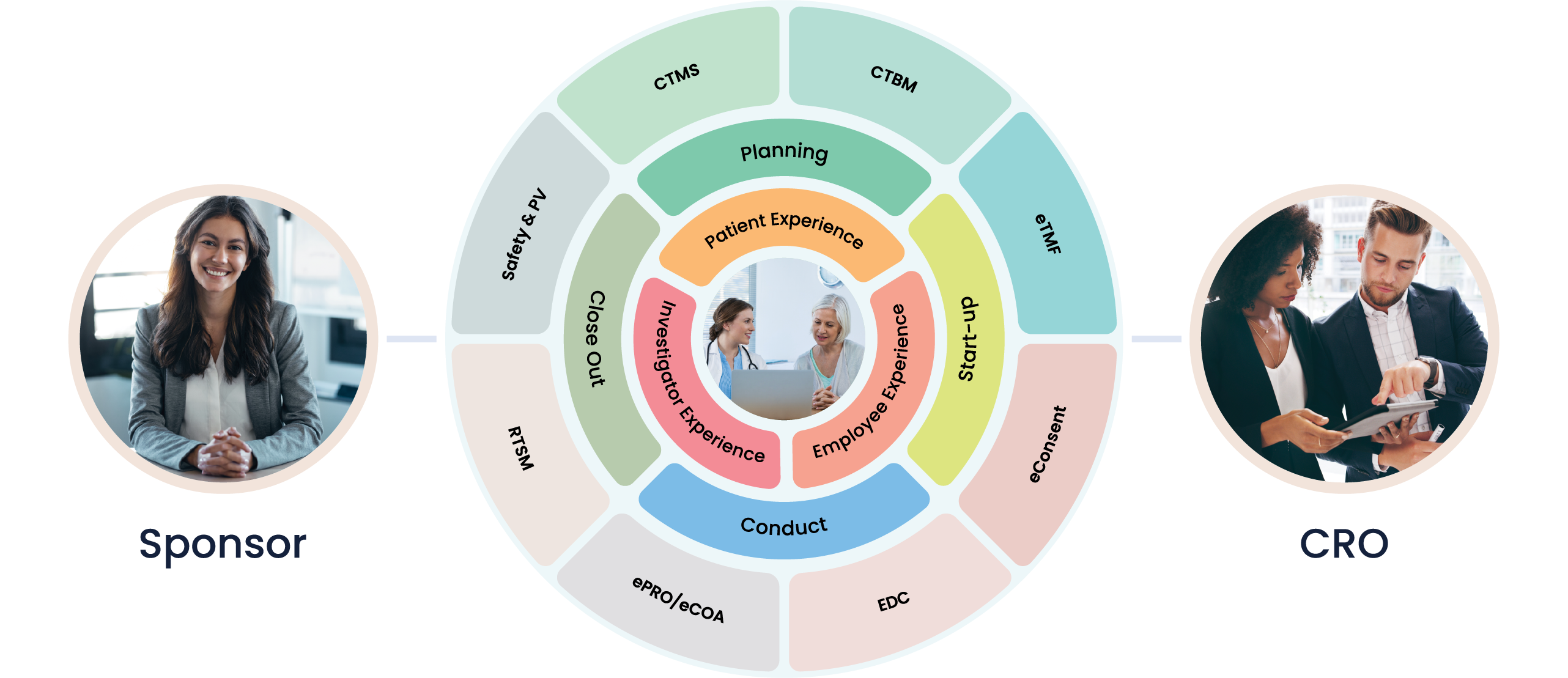
At Cloudbyz, we understand the pivotal role clinical trials play in the breakthrough field of cell and gene therapy. We acknowledge the unique challenges this industry faces, and we've tailored our solutions to streamline these processes, fostering efficiency and delivering reliable, actionable results.