TRUSTED BY
Investigator Portal
Clinical trial sponsors and research sites are required to use many different websites and maintain multiple login accounts to perform clinical trial responsibilities and communicate with study sponsors. Investigators and site personnel spend hundreds of hours performing redundant tasks across multiple sponsors and systems. Most trial technologies hinder investigator efficiency and breed frustration.
Cloudbyz Investigator Portal provides a single platform to deliver content and services to Investigator Sites, and provides a single point of access for interaction with participating sites.
Product Features
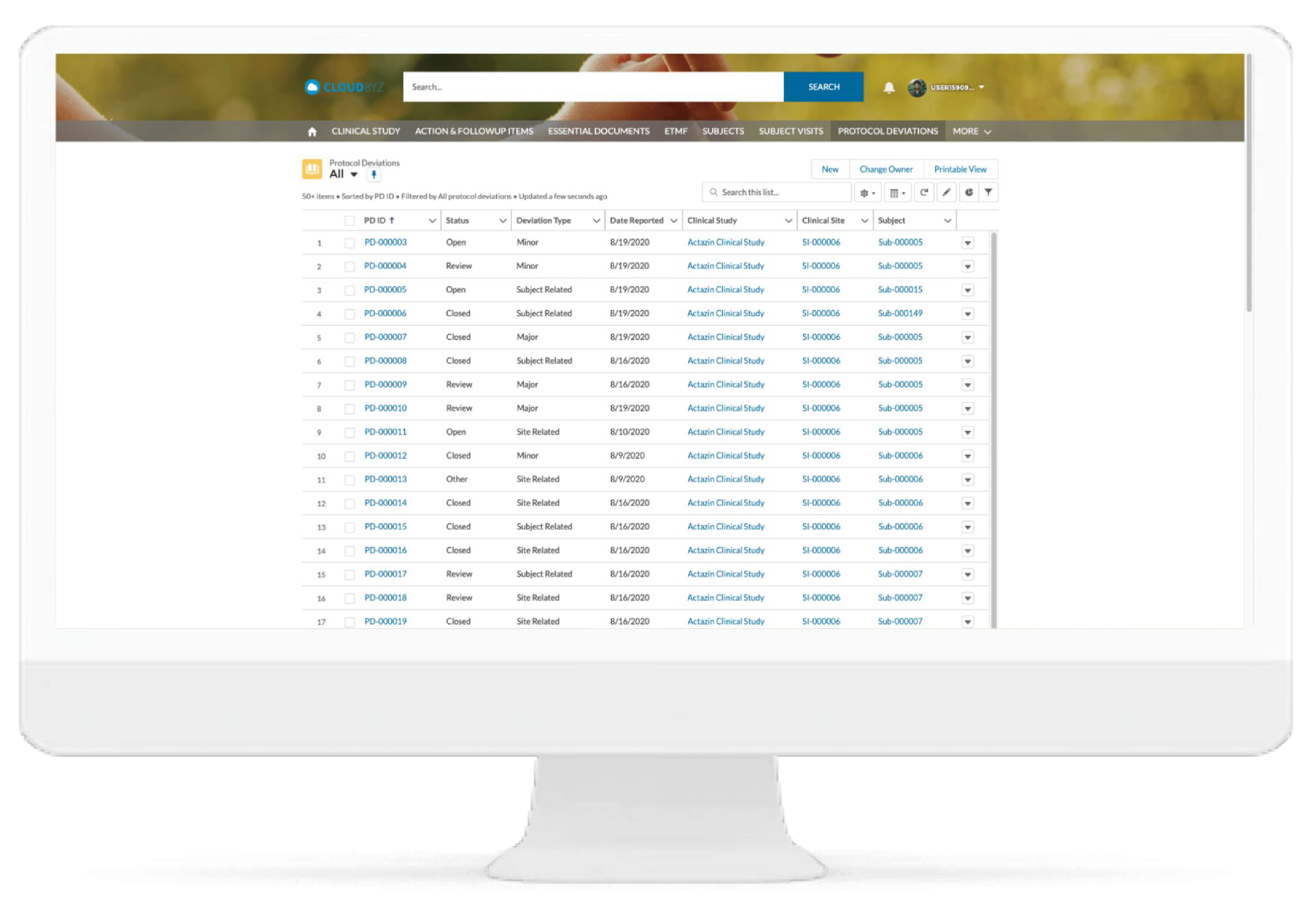
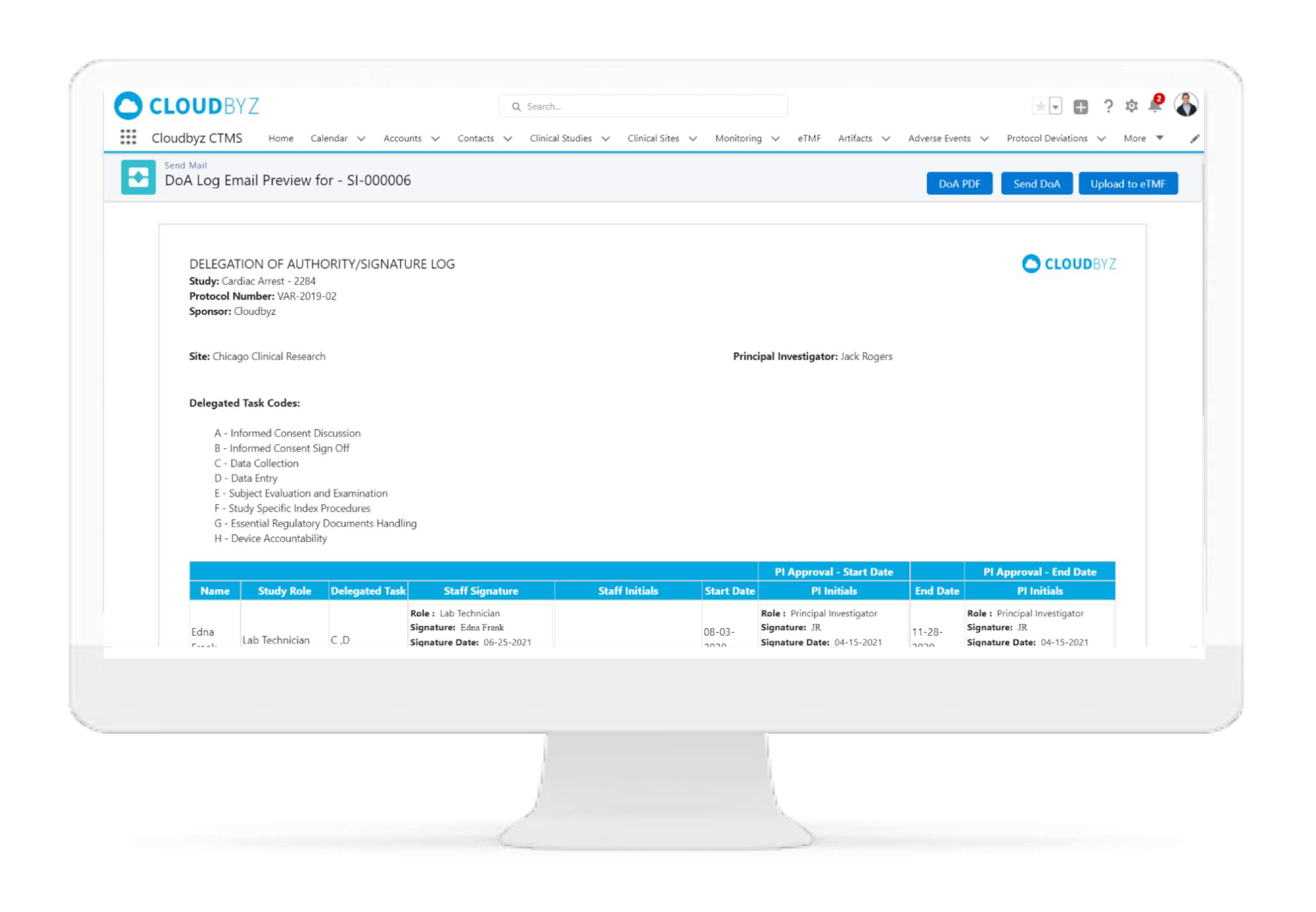
Delegation of Authority(DOA) Log
Delegation of Authority (DOA) Log
Cloudbyz has completely remodelled the DOA log process. With study team members working remotely, maintaining the paper based DOA log is particularly challenging.
Through the Cloudbyz system, you can build your own delegation log, create the study specific roles and responsibilities, and easily assign these responsibilities based on the study role. Have your site team sign the DOA log electronically directly from the system and upload completed DOA to the eTMF. For sites who prefer wet ink signatures, the DOA log can also be generated in a PDF/ printable version and sent to the sites via email directly through the system.