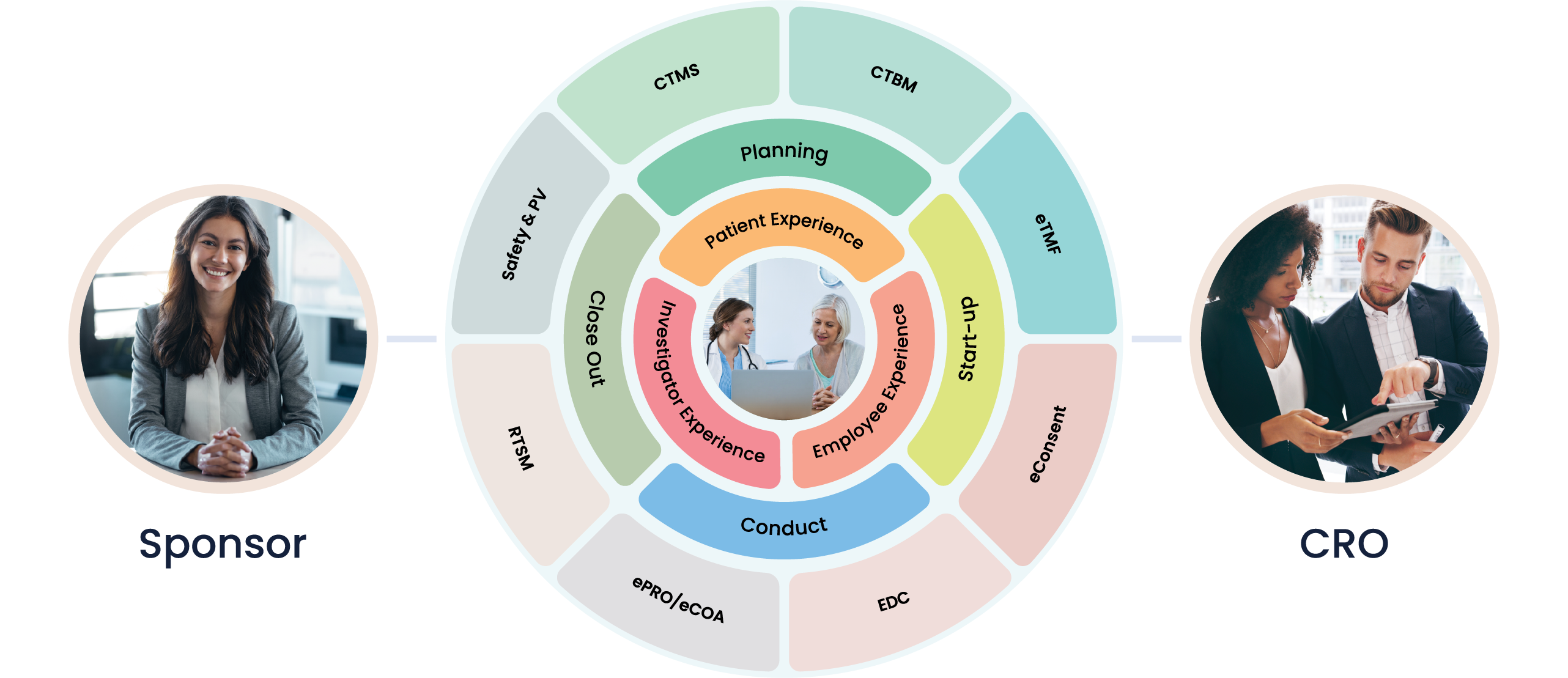
At Cloudbyz, we are deeply committed to driving innovation in the medical device industry. We understand the intricacies and complexities of conducting clinical trials, and our solutions are designed to simplify the process, thereby enhancing efficiency and promoting faster time-to-market for breakthrough medical devices.