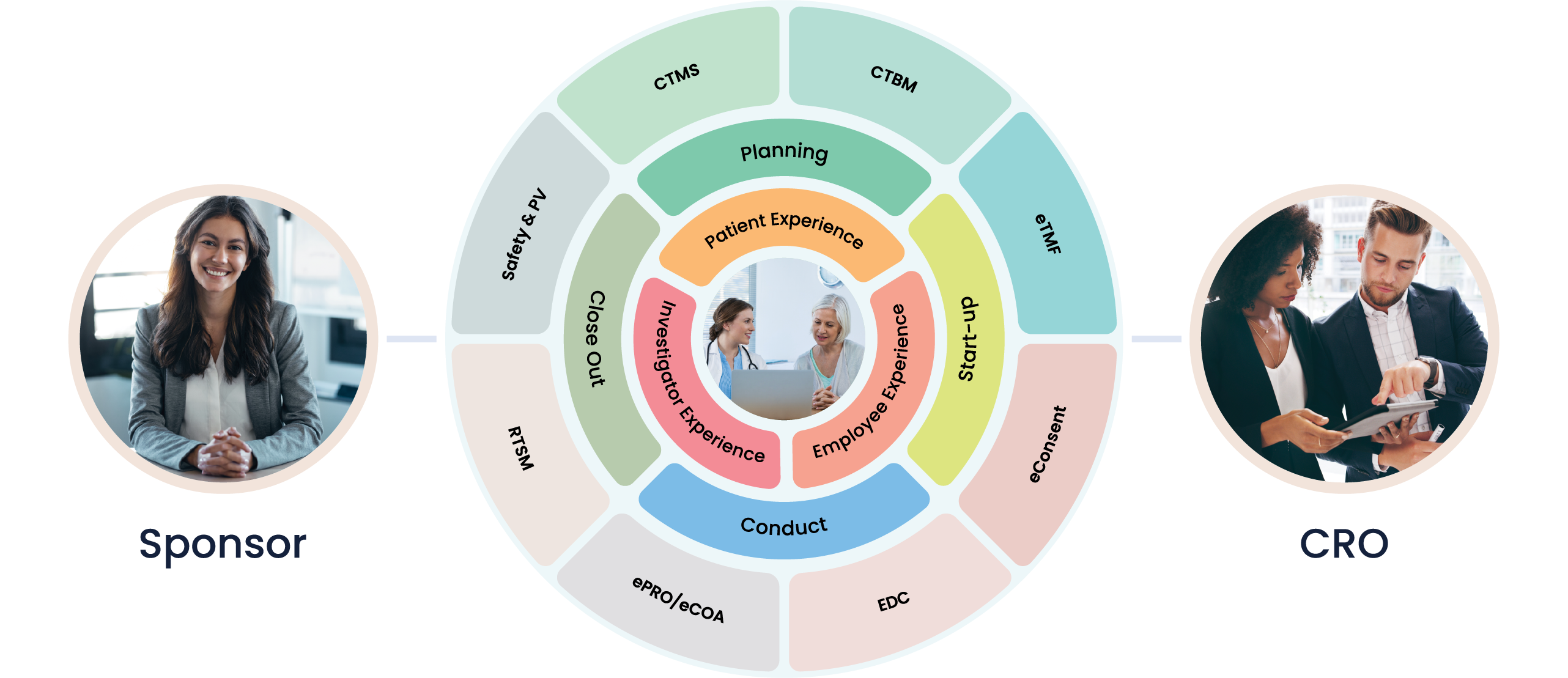
Digital therapeutics is a fast-paced and innovative industry where rigorous clinical trials are crucial to ensure the efficacy and safety of new digital health interventions. Cloudbyz offers a comprehensive and specialized clinical trial management solution to meet the distinct challenges of this emerging field.