TRUSTED BY
Cloudbyz built the Study Start-up solution on the Salesforce Cloud platform to accelerate study start-up and activation. The solution provides the ability to: track site performance metrics, profile, perform feasibility surveys and via Portal, provides a centralized location for accessing and sharing all study-level information. Registration process for CRA and investigators to participate in the study and cases for inquiry management. Chatter based collaboration forum to discuss and collaborate around study between investigators and study coordinators. Directories with contact information for all study personnel including external vendors. Study library serves as a repository for study-related training tools and documents. Study news updates and alerts. Supply order/reorder forms.
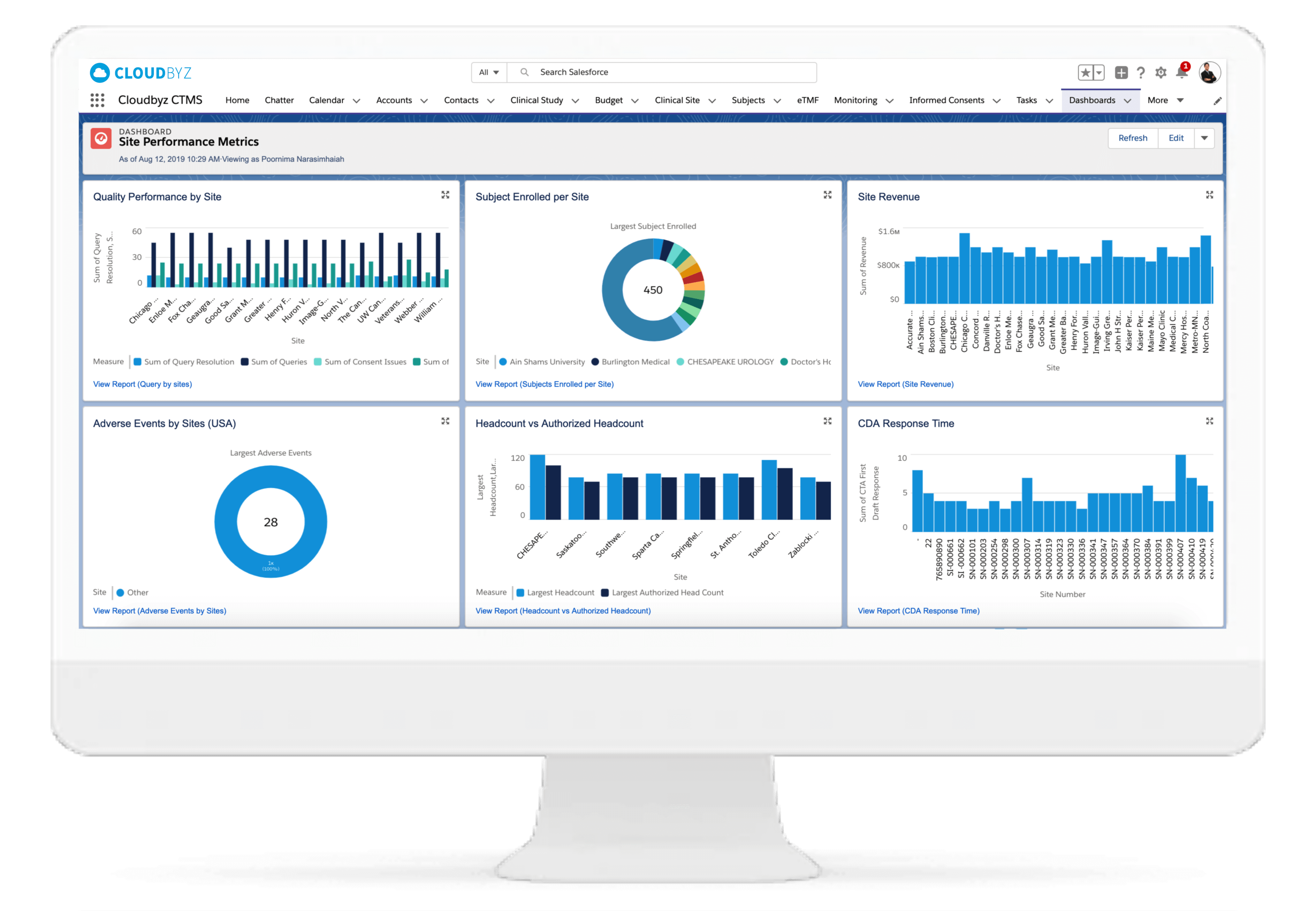
The site performance metrics in this portal can help in better tracking of site details including equipment, staff, and revenue and performance metrics related to previous trial process.
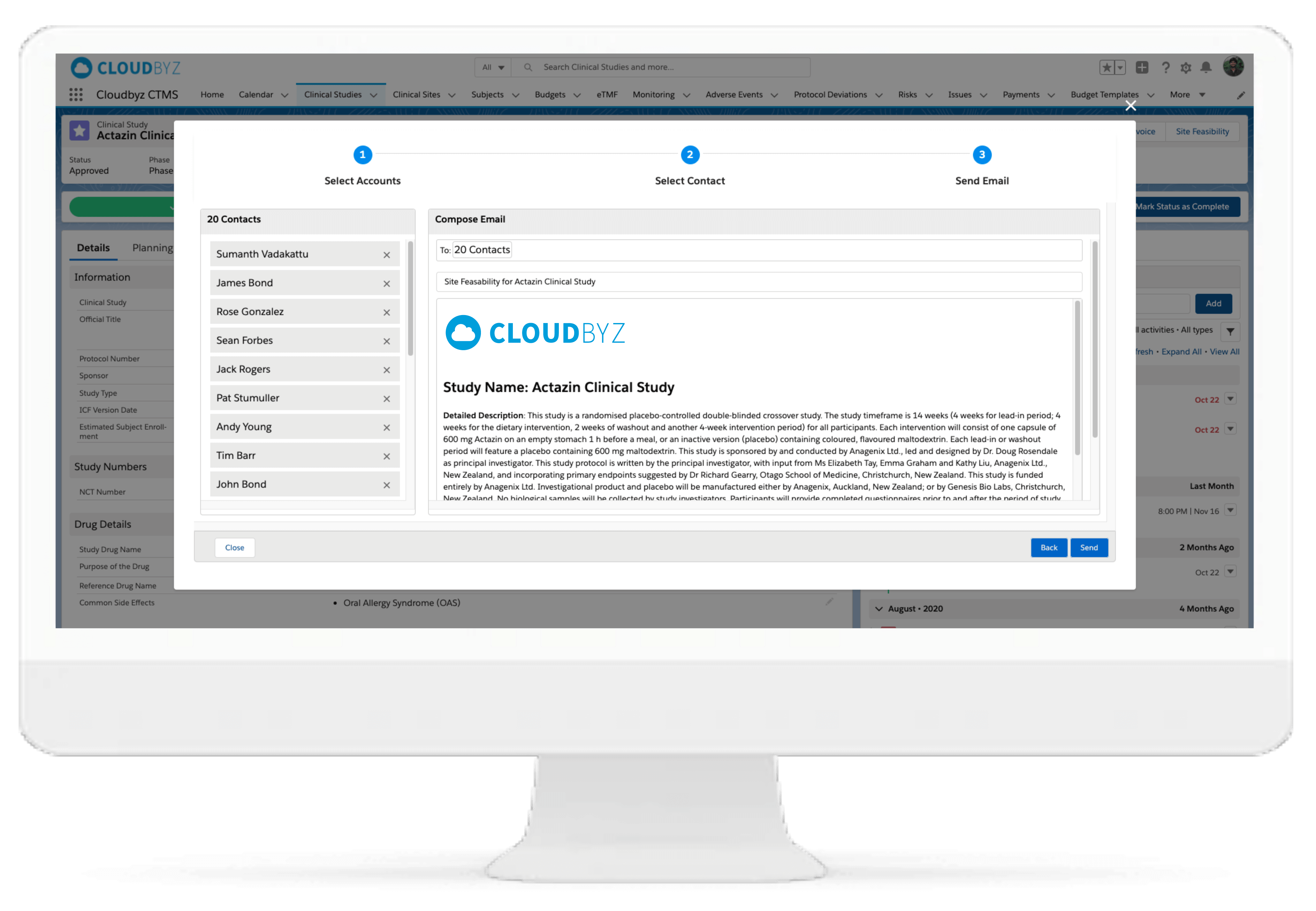
This is an assessment tool to evaluate the feasibility of sites, potentially to be used in clinical trials. The end objective is successful, cost-efficient, on-time trial project completion.
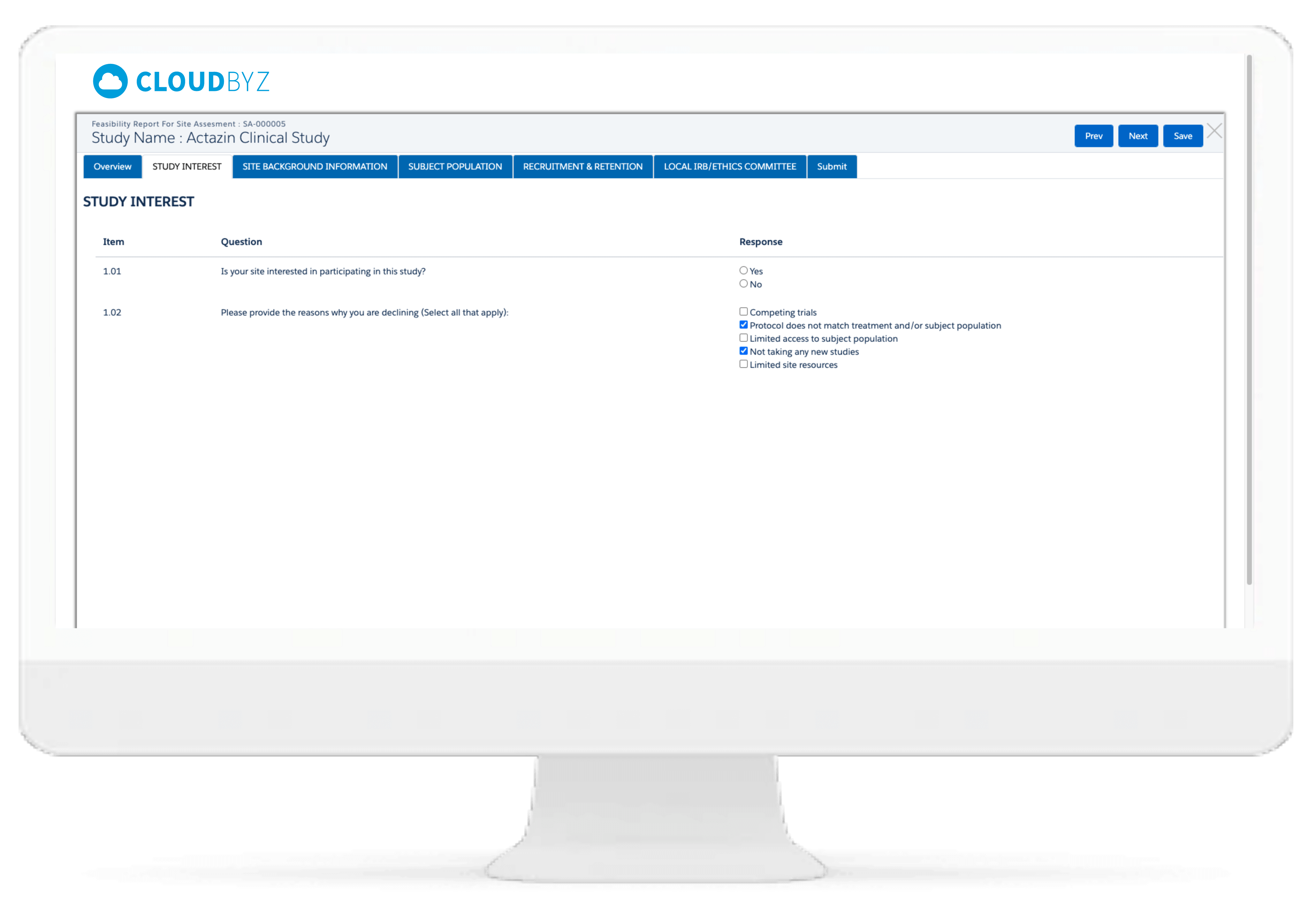
Users can create feasibility templates and conduct online feasibility assessment surveys alongside real-time access to survey responses for faster feasibility evaluation and site activation.
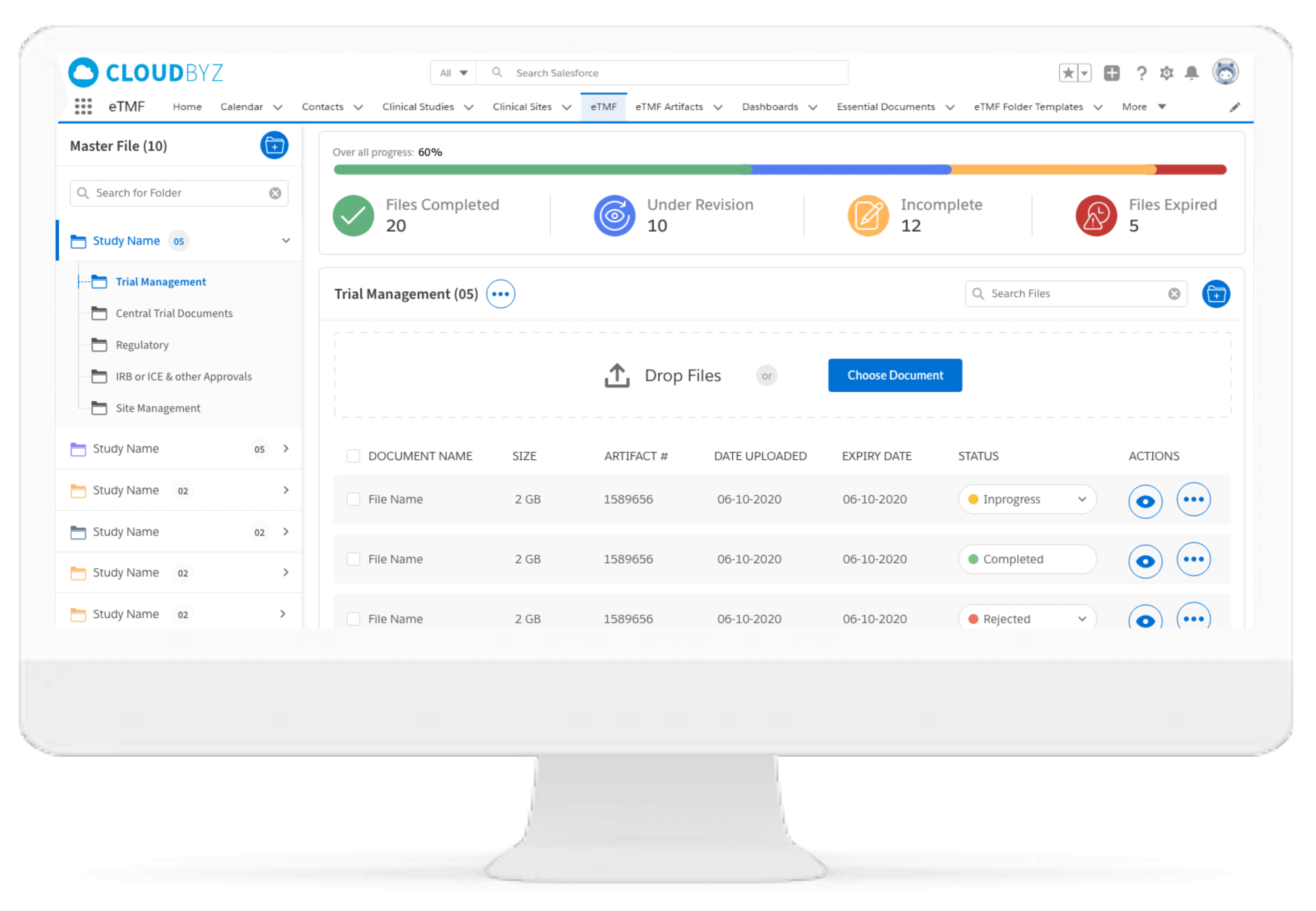
The document management functionality can help in dissemination and review of significant documents for helping participants in using latest version of study documentation thereby ensuring relevance.
Cloudbyz study start-up software is a cloud-based Salesforce built solution to enable a centralized repository of all study level information along with accelerated studu activation and collaboration between the involved clinical trial stakeholders like CRO, sponsors and site.