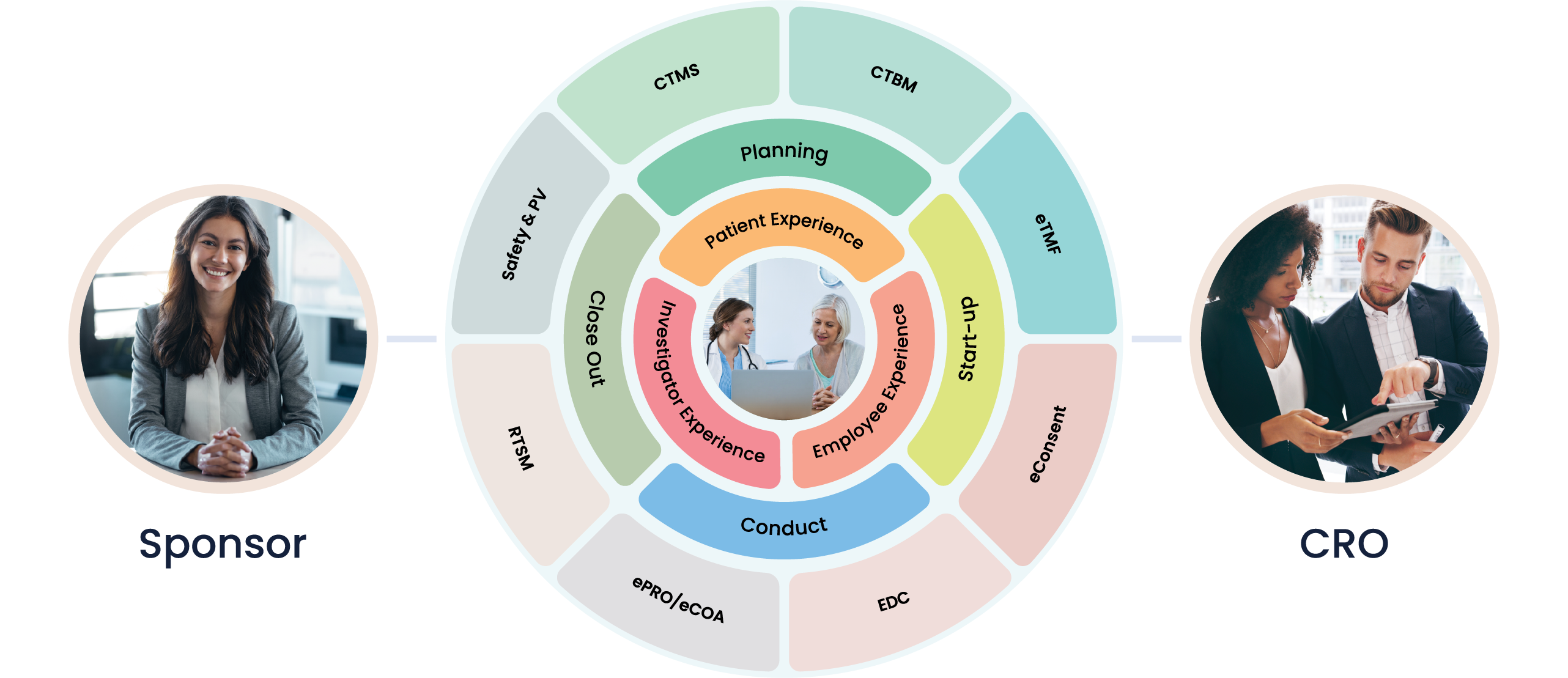
At Cloudbyz, we recognize the critical role clinical trials play in the diagnostics industry. Understanding the unique complexities associated with these processes, we've tailored our solutions to ease these tasks, promoting efficiency and delivering precise, actionable results.