-
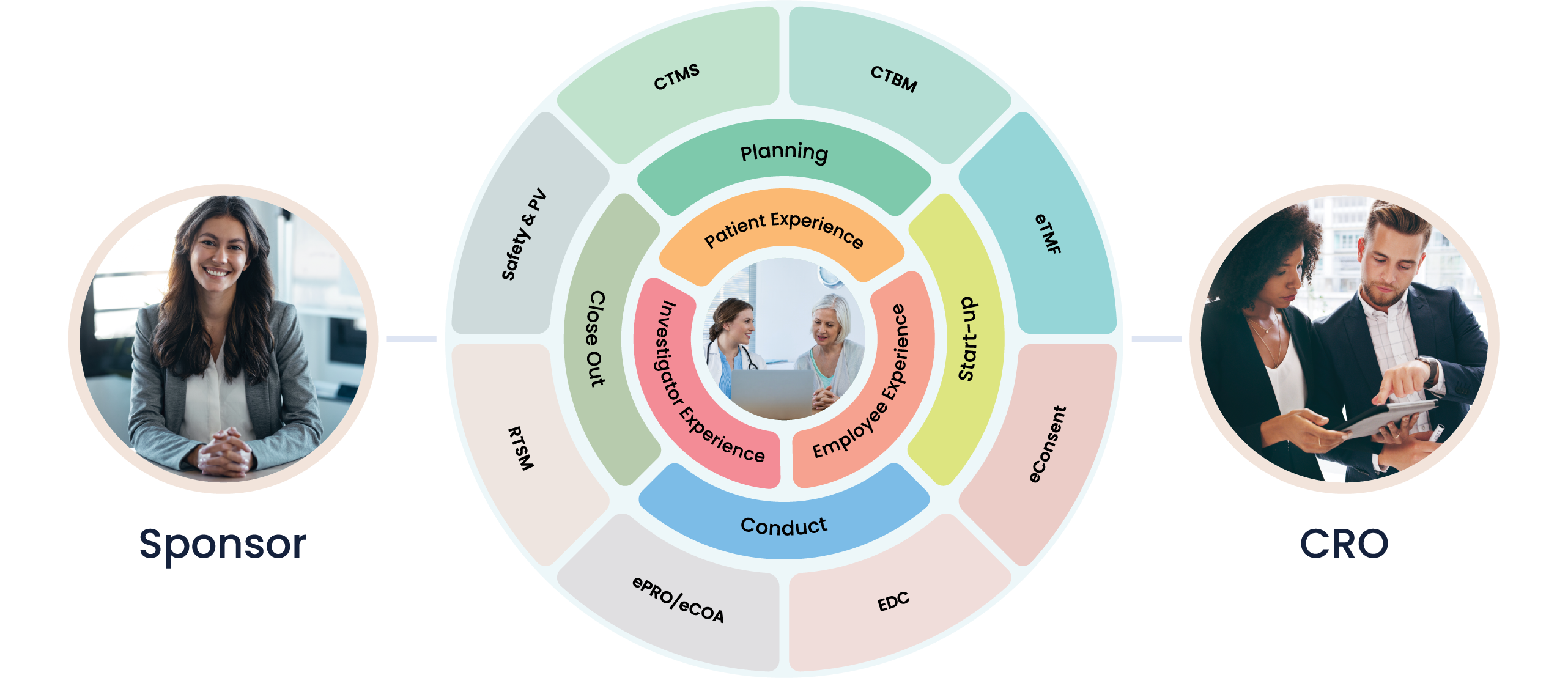
Clinical Trial Management System (CTMS)
Manage clinical trial operations, collaborate with study partners, and view real-time data all-in-one place.
-
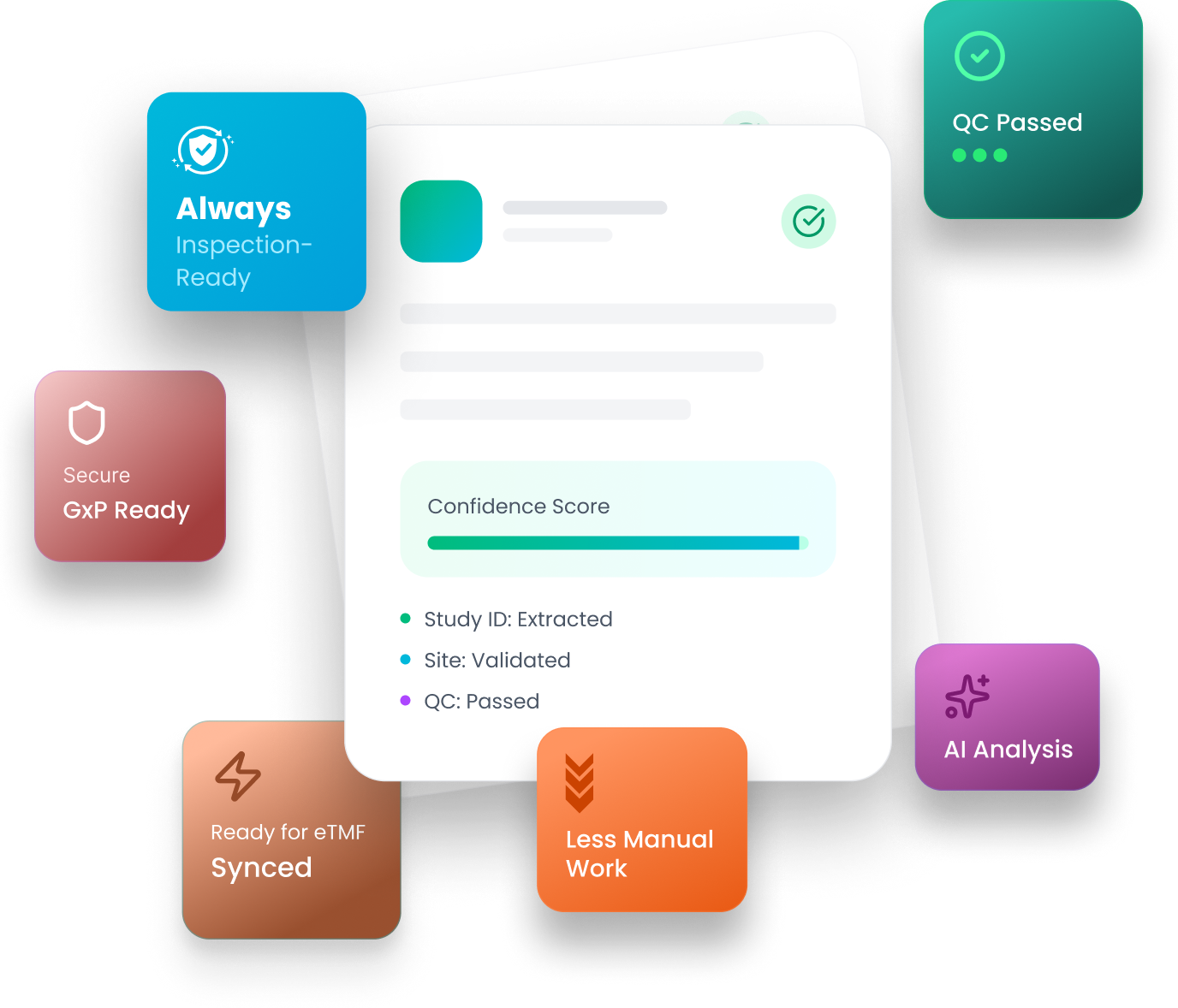
Electronic Trial Master File (eTMF)
Manage essential trial documents, stay inspection ready, and collaborate in real-time with study partners.
-
Electronic Data Capture (EDC)
Collect data in real time, reduce redundancies, and enhance data quality.
-
Safety & Pharmacovigilance (PV)
Manage all Safety and PV processes with a robust, secure, and compliant pharmacovigilance platform.