TRUSTED BY

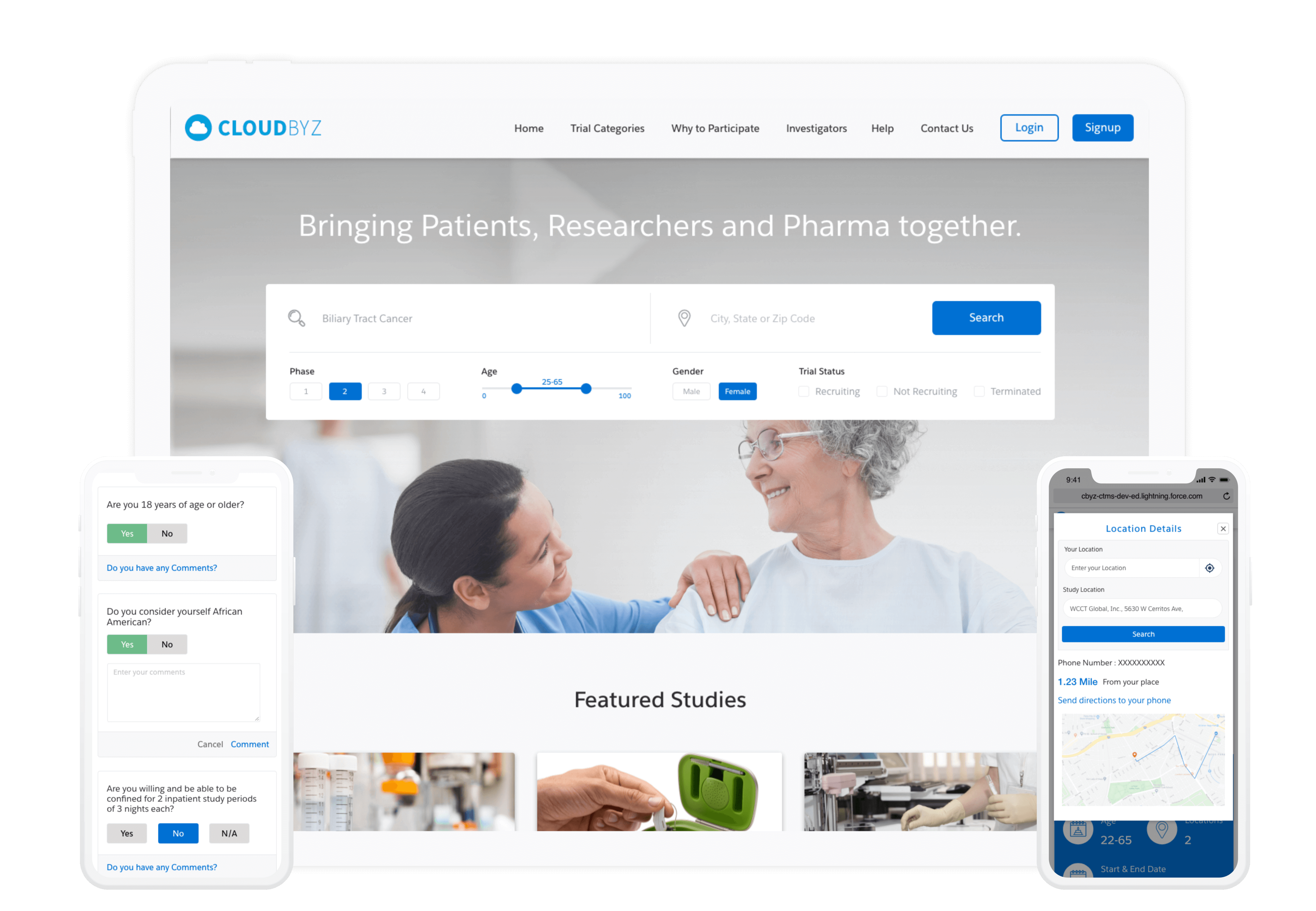
Cloudbyz offers a cloud-based clinical trial management solution built natively on Salesforce, to cater to every clinical trial requirement in the healthcare and life science industry.
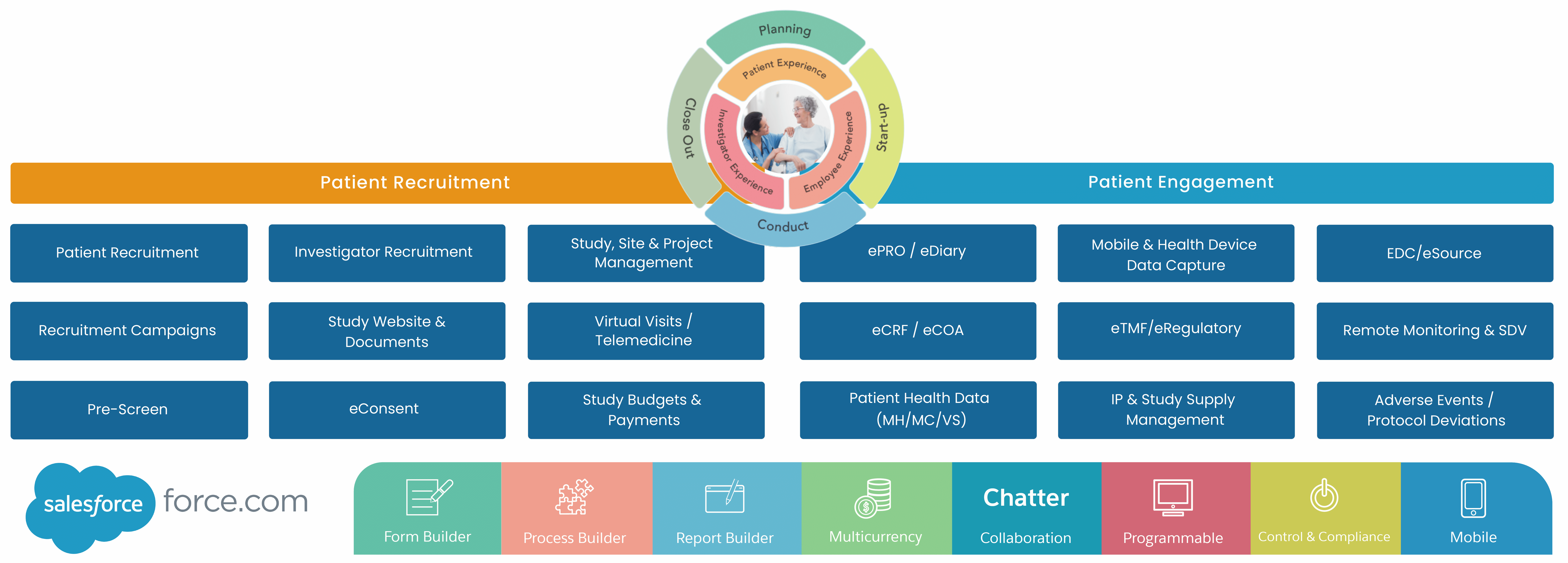
Cloudbyz pioneers in providing digital capabilities and clinical trial management solutions (CTMS) across the clinical trials lifecycle with patient centricity at its core. Our decentralized clinical trial (DCT) portfolio including smart features for eConsent, eCRF, EDC, ePRO, remote monitoring & SDV, eCOA, eDiary, virtual PR, eTMF, among others.










Cloudbyz investigator recruitment, a pioneer in this functionality, enables investigators to gain easy access to feasibility study of any existing/potential site with regards to clinical trials. We facilitate mass email distribution (using the investigator database) targeted to different investigators with accessible site feasibility links.





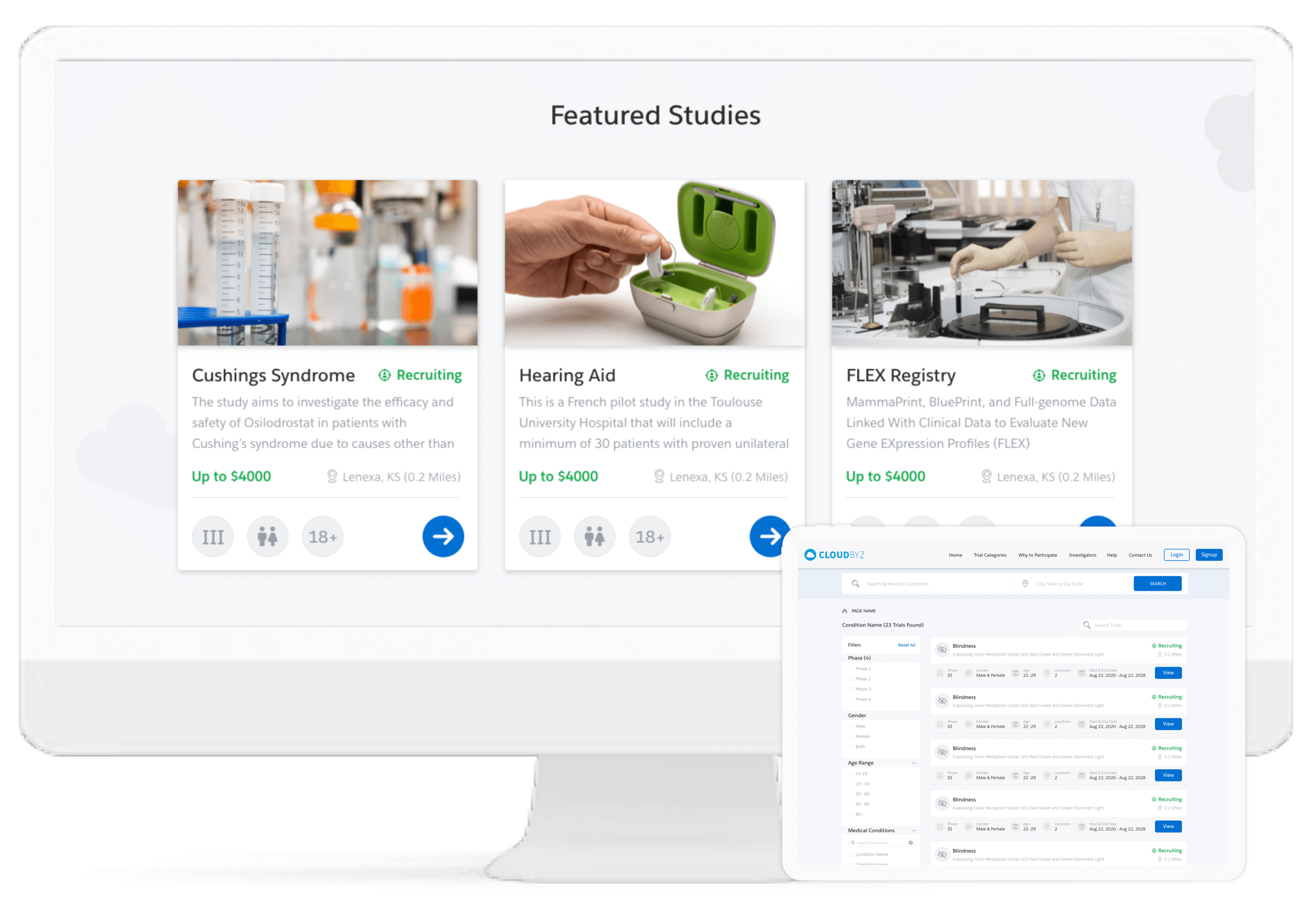
Cloudbyz enables comprehensive study, site and end-to-end project management of the clinical trials through its different CTMS modules. It delivers complete off-site and hybrid-based process streamlining including site feasibility study, report monitoring, study management, IRB approval tracking, training, real-time performance tracking, among others.





Cloudbyz supports virtual visits for investigators/CRO and patients by generating visit appointments as per the schedules and maintaining schedule calendar. Telemedicine functionality is used for routine follow-ups to identify adverse events. It also helps in setting up procedures, subject visits and payment policies depending upon the budgets and study protocols.





Cloudbyz DCT budget solutions enable a hawk-eye budget estimation, planning, payments management across clinical trials. Cloudbyz delivers defined budget templates, real-time tracking of investigator grants, procedures and visit payments along with budget projections and variance between planned and realized expenses.





Cloudbyz decentralized clinical trial solution enables capturing patient data electronically both from wearables and manually entered information by investigators. Patient health data (like heart beat, SpO2,etc) from multiple gadgets, on a real-time basis are extracted through integrations. Also other data like medical history can be updated and rolled-up post manual feed on the participant portal.










Cloudbyz solution offers electronic data capture (EDC), with product features for source data validation (SDV), questionnaire templates, queries and eSignature and transcribes them into systems. It facilitates faster data processing and centralized maintenance of clinical patient data.





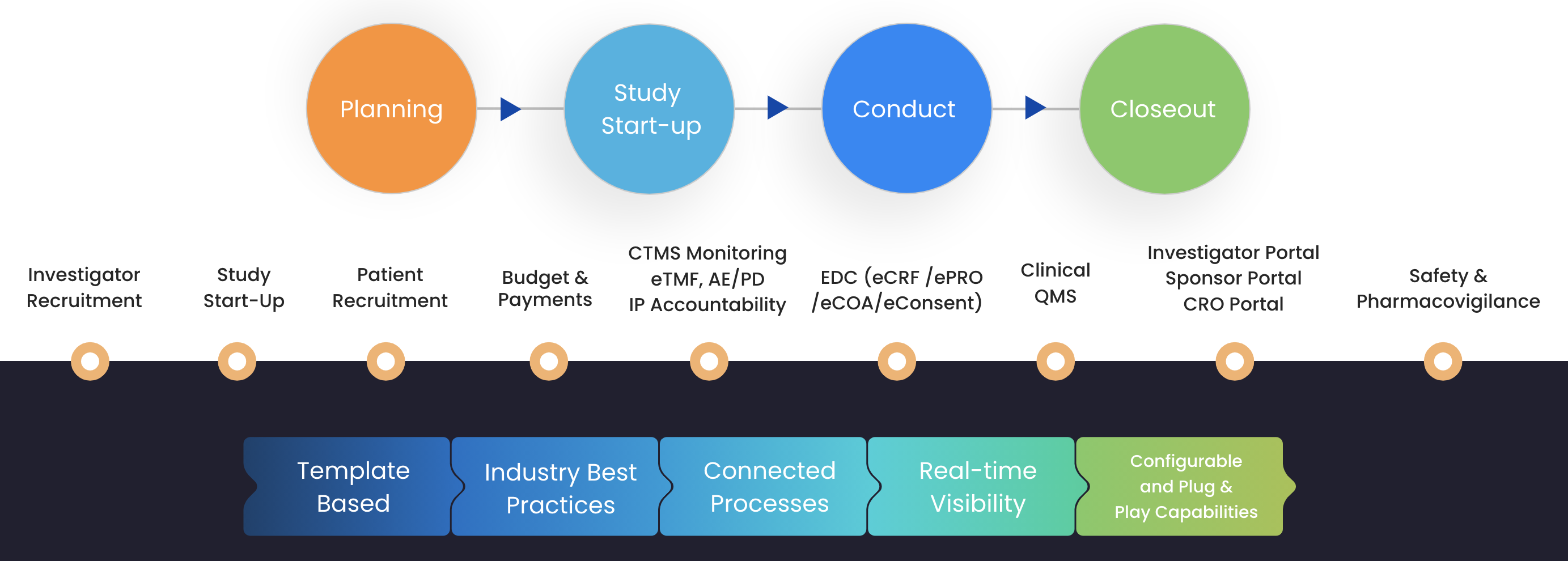
Cloudbyz decentralized clinical trial solutions (DCT) serves as a digital Salesforce built solution to almost every decentralized trial related requirement across the clinical trial lifecycle. Our customer-centric cloud based approach within the Cloudbyz clinical trial management solution, is embedded across our decentralized clinical trial portfolio to offer its users a competitive edge with the digital era and the prevailing virtual clinical trial ecosystem. This Salesforce decentralized clinical trial solution offers agility, flexibility, and scalability for its users including CROs, sponsors, sites and every stakeholder of the clinical trial study.