TRUSTED BY
eRegulatory
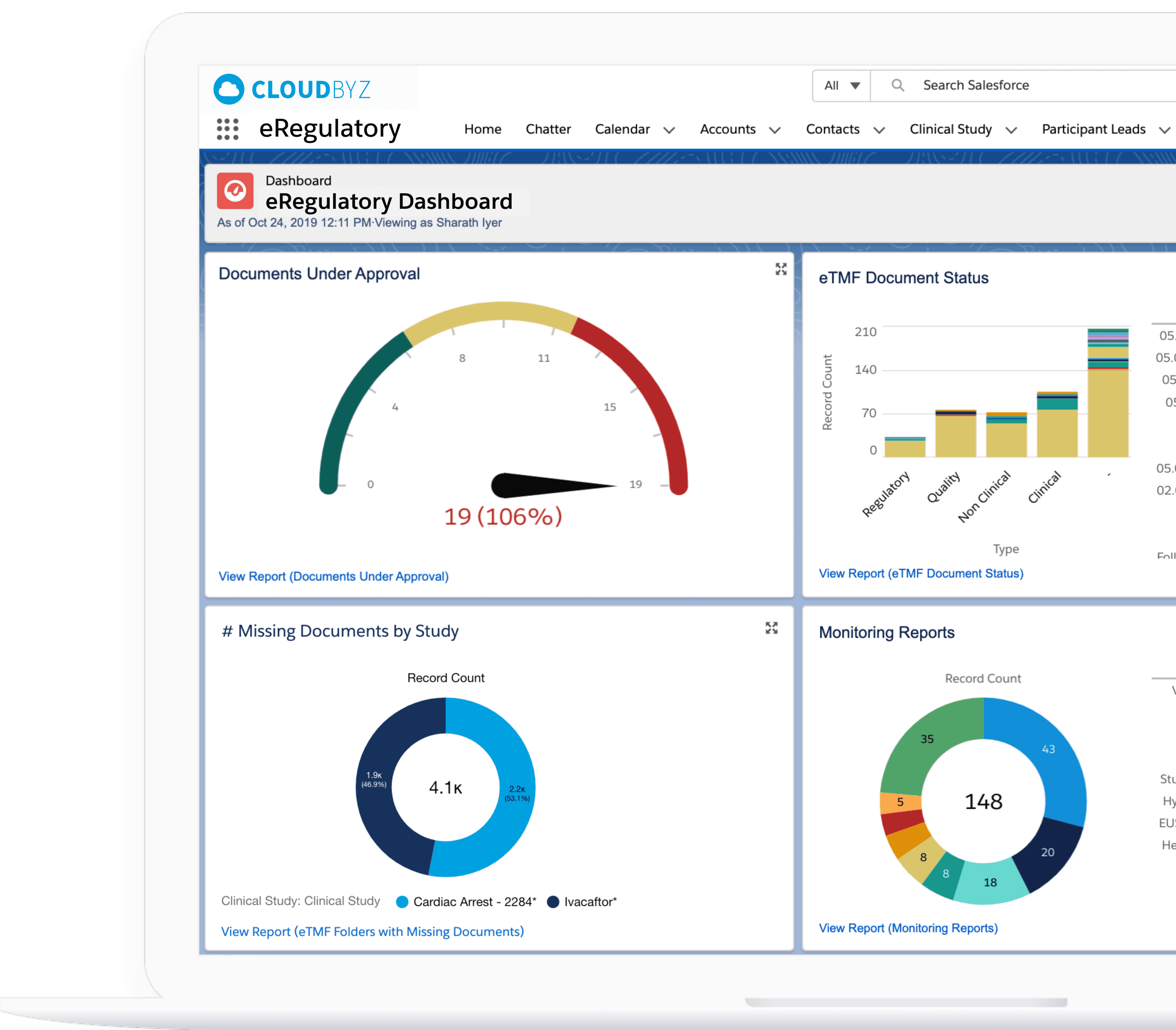
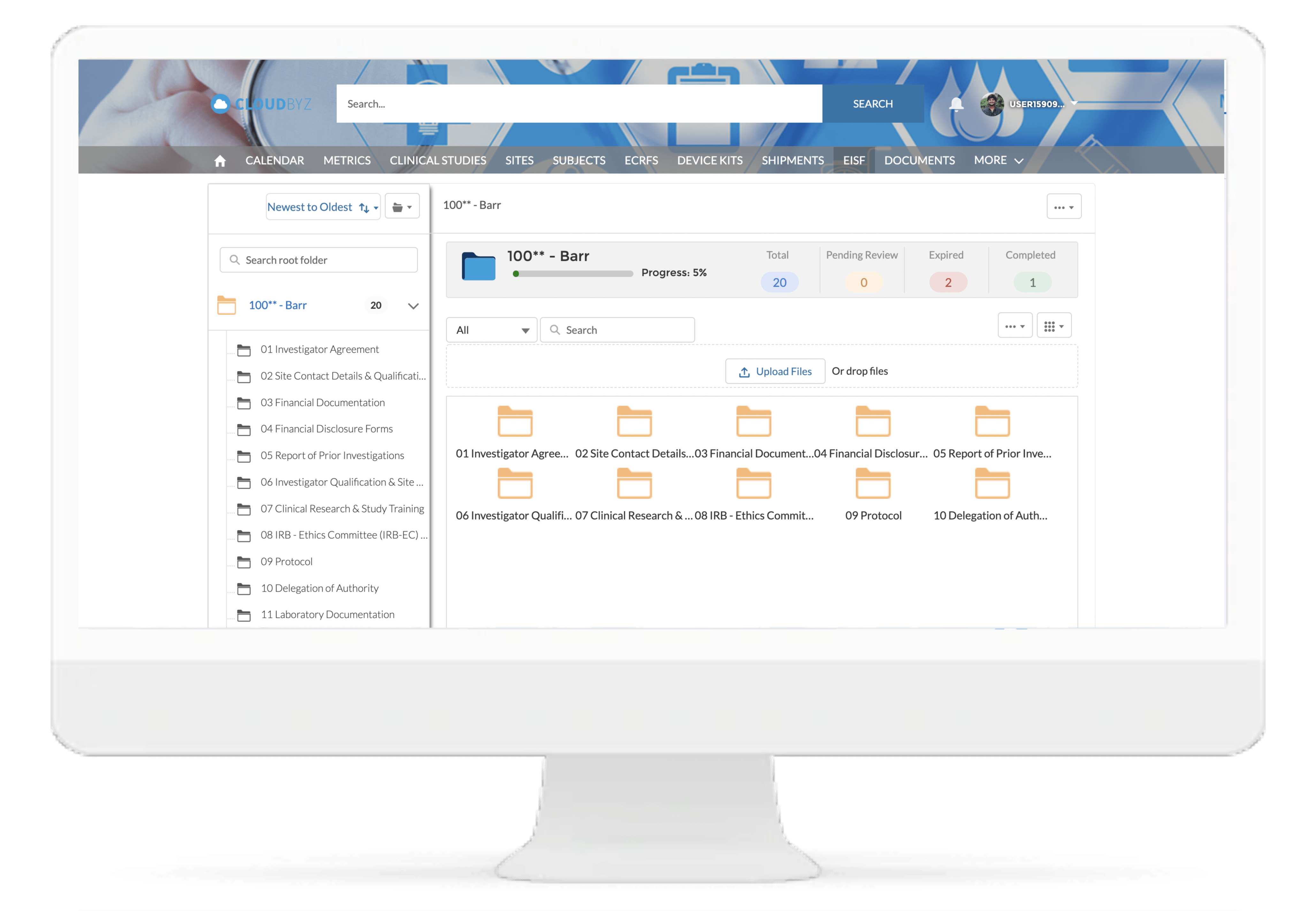
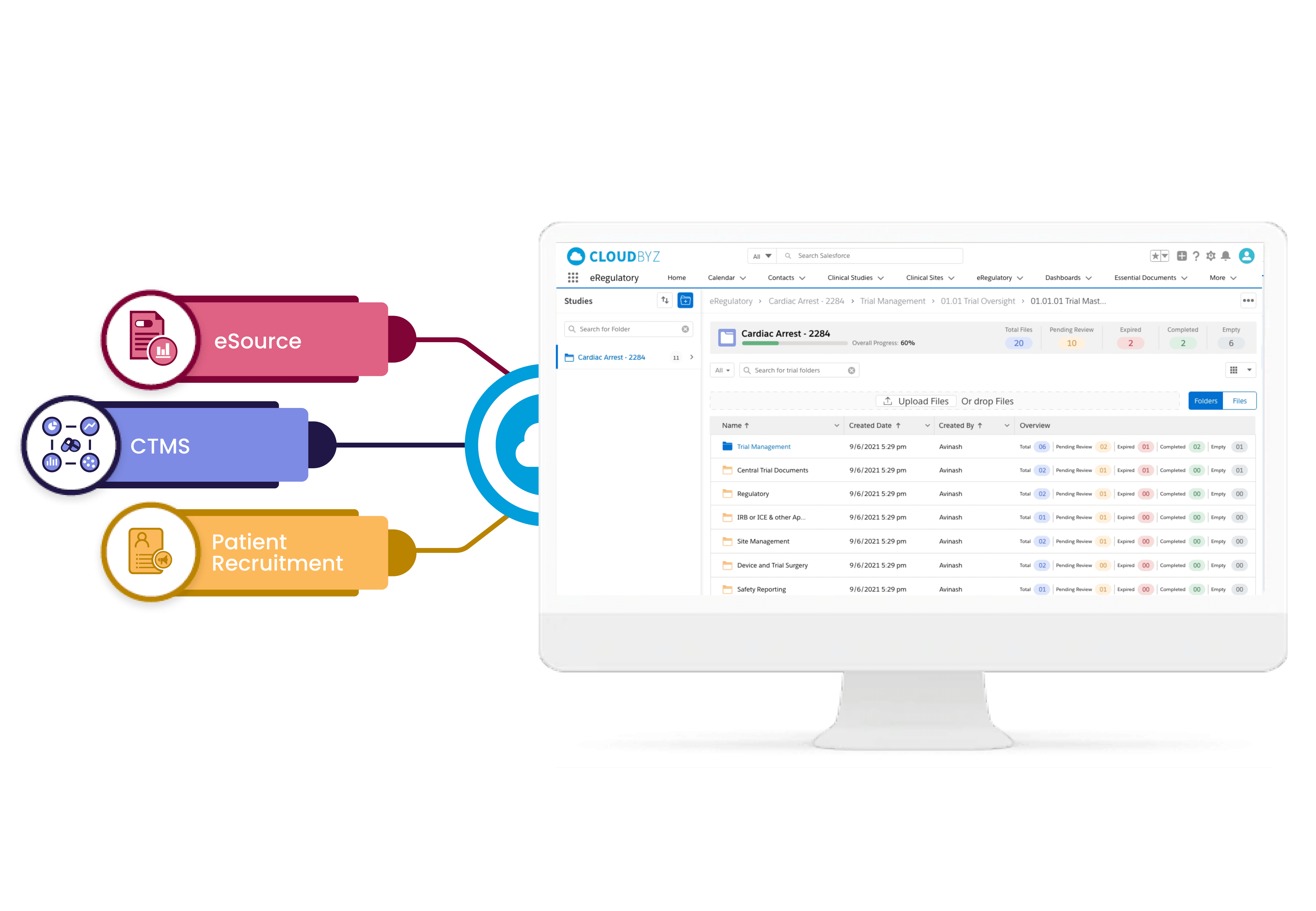
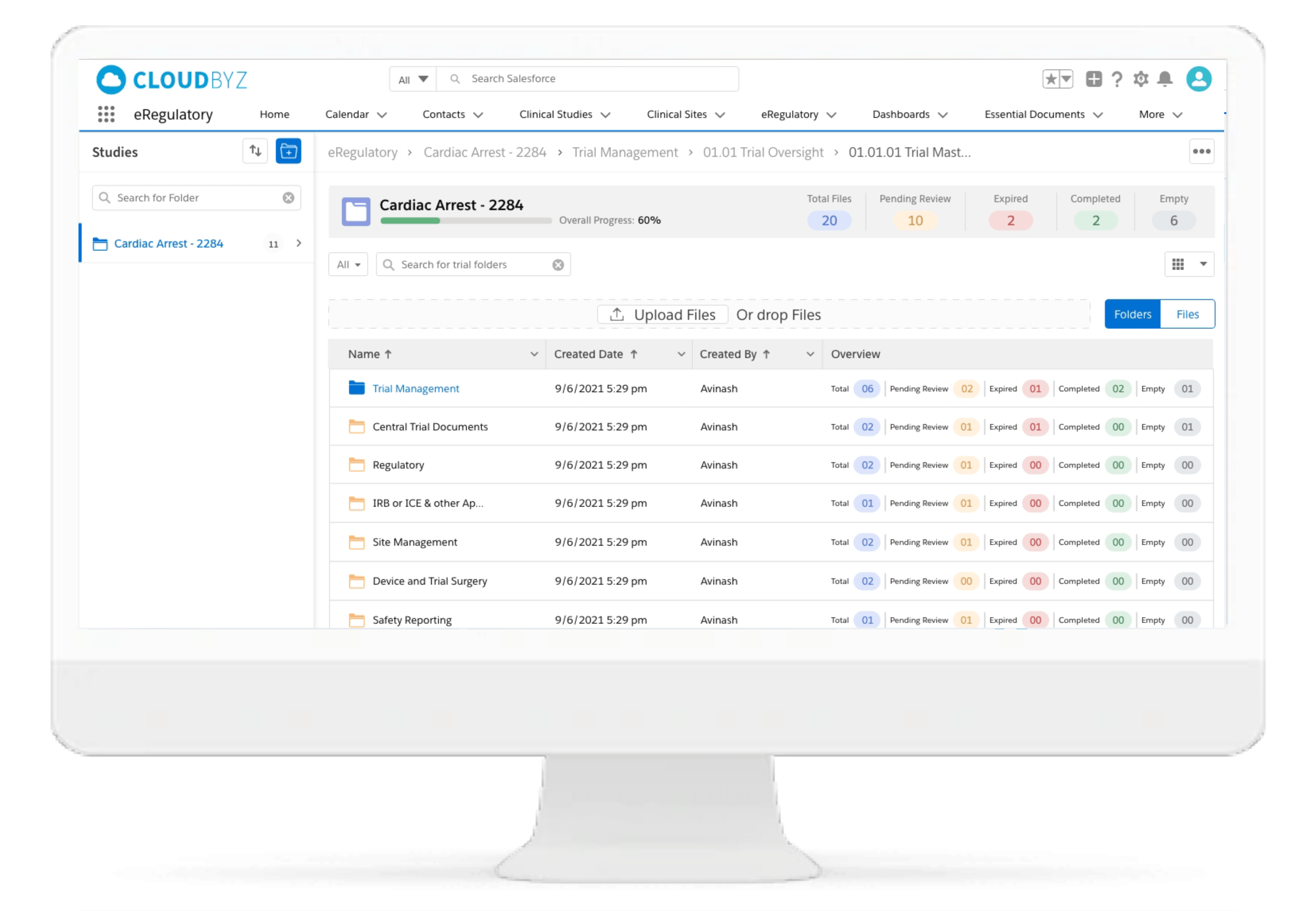
Cloudbyz eRegulatory solution offers a cloud-based repository for all of your clinical trial documents. Digitally capture, manage, share, and store all clinical trial-related documents with a centralized overview. Manage essential trial documents, stay inspection ready, and enable real-time visibility for CROs, sponsors, monitors, and other stakeholders in a clinical trial.
With Cloudbyz eRegulatory, research sites can streamline regulatory workflows with paperless binders and an electronic delegation log.
Product Features
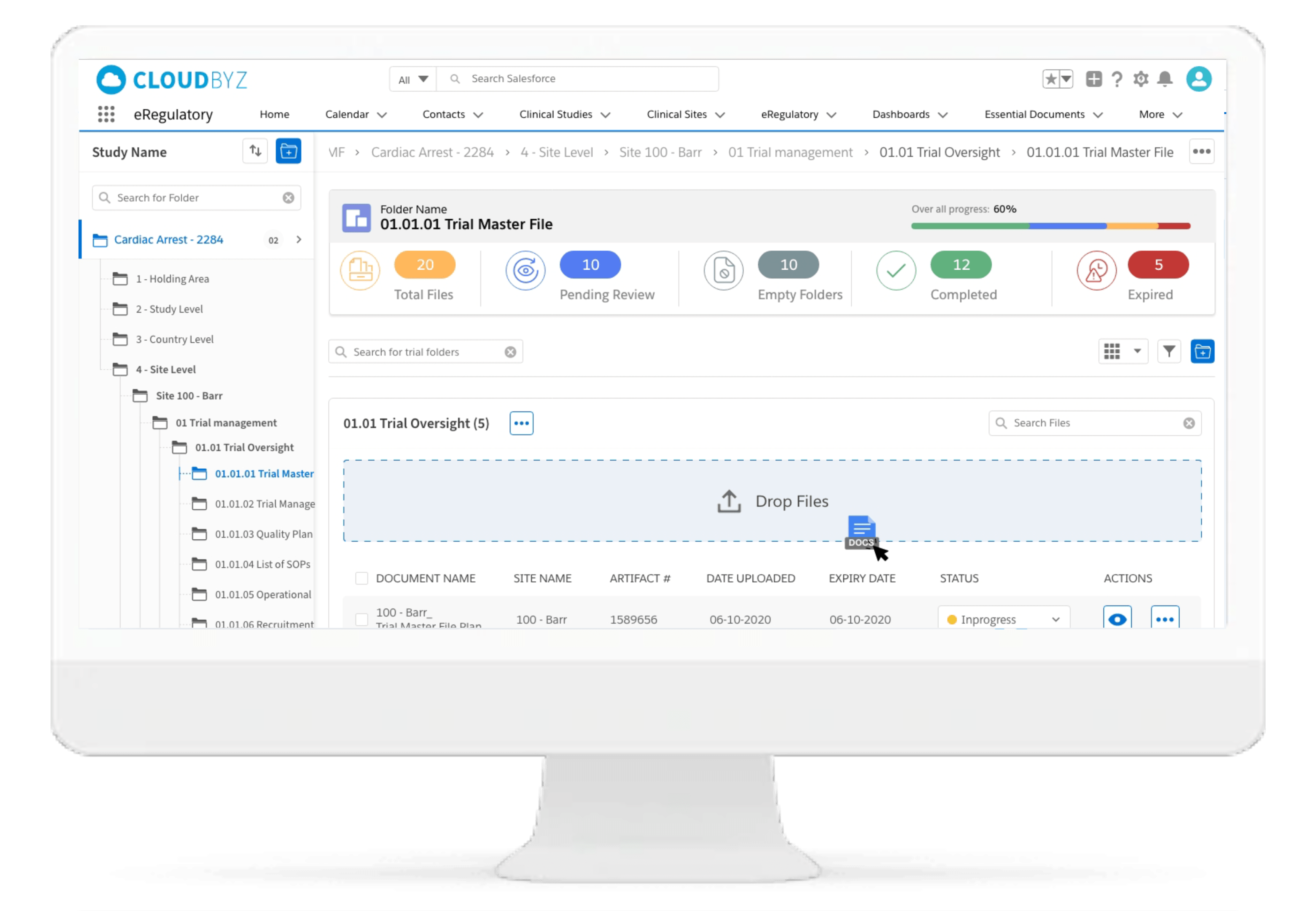
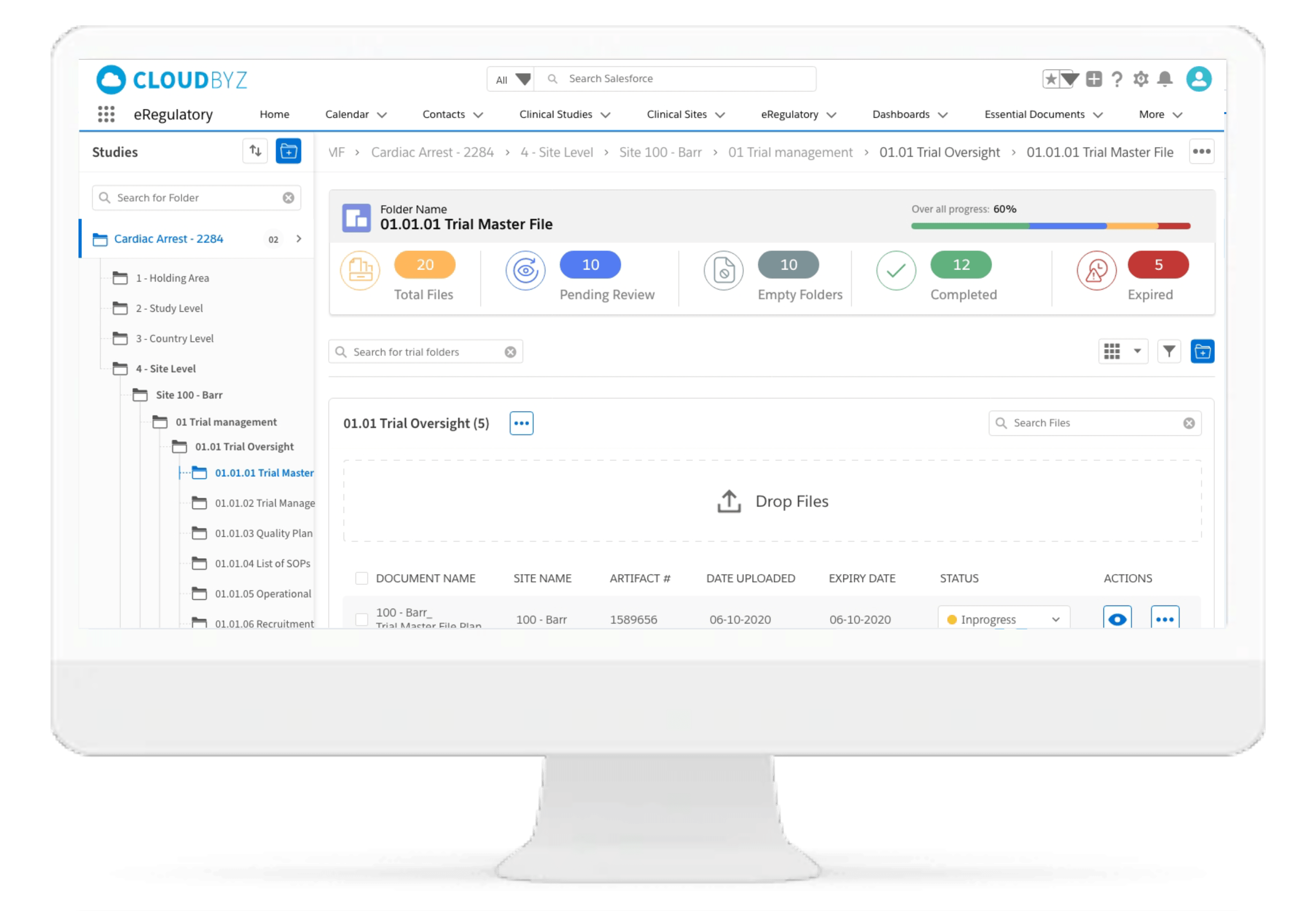
Centralized File Management
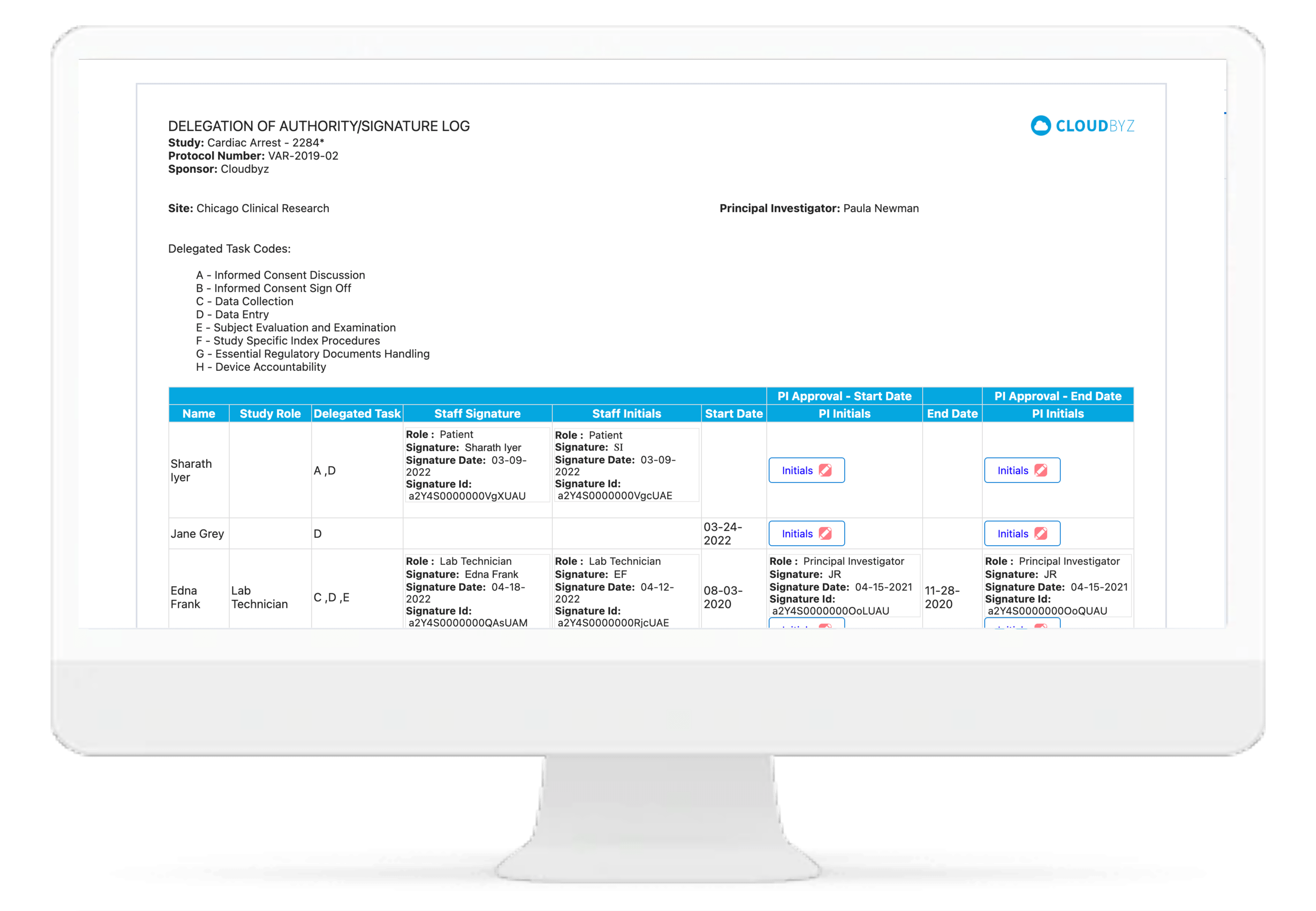
Electronic DOA
Electronic DOA
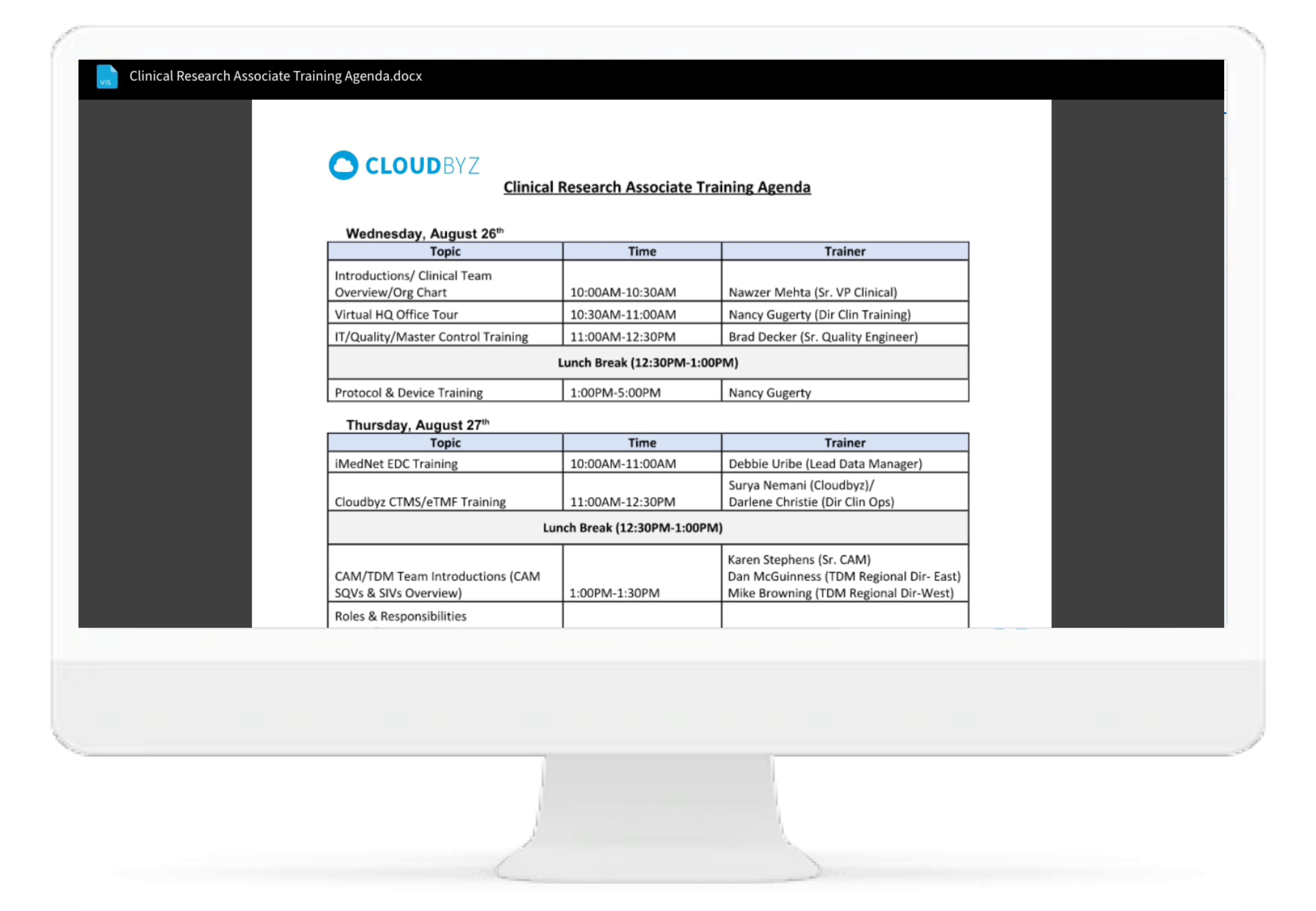
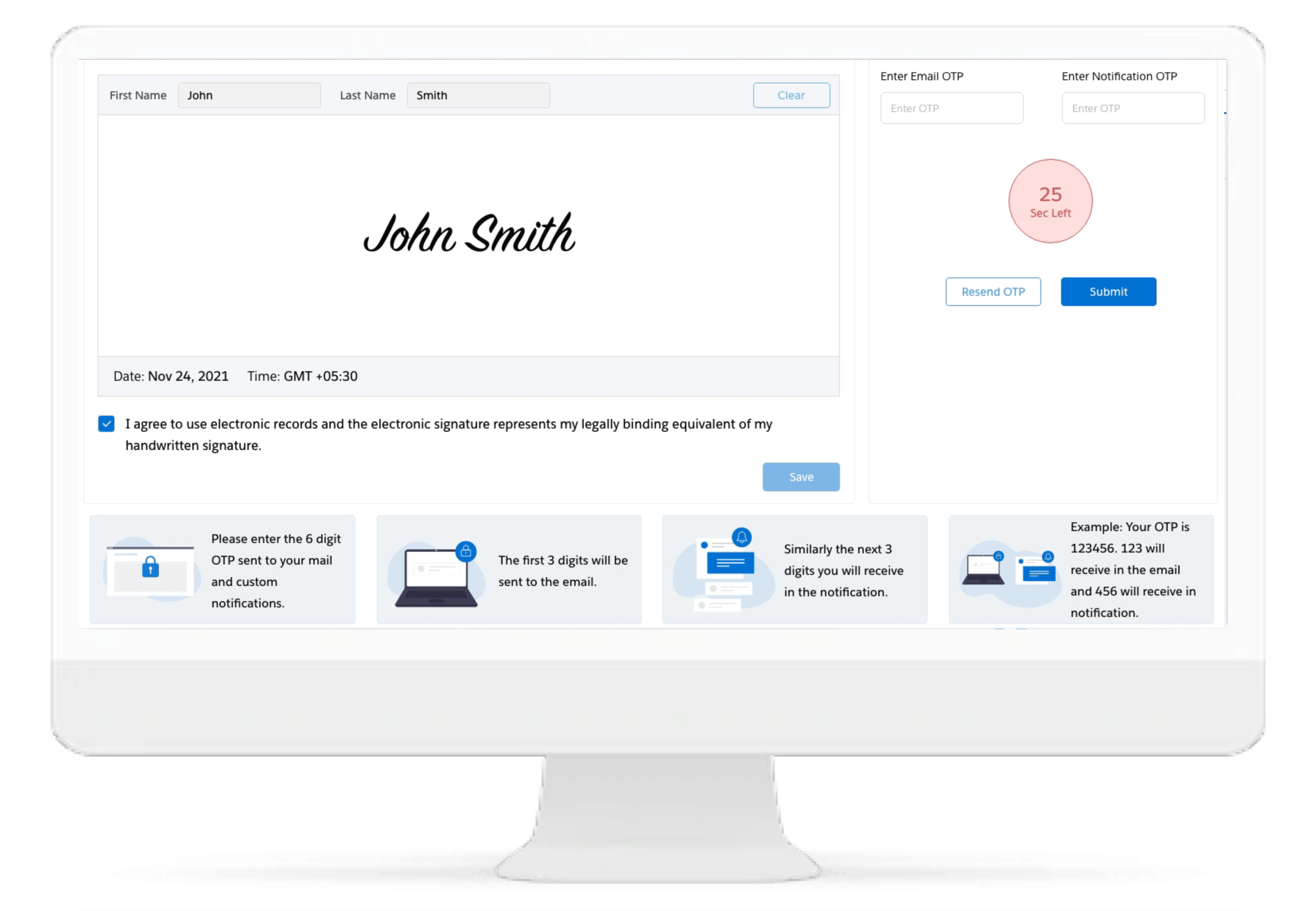
Cloudbyz eRegulatory solution comes with automatic generation of electronic delegation of authority log (eDOA) based on the team roles and assigned responsibilities. The study team can sign on the eDOA log through inbuilt electronic signatures. The log can be maintained live for the entire duration of study and can be generated for filing into the site binder and/or the eTMF.
Key Benefits
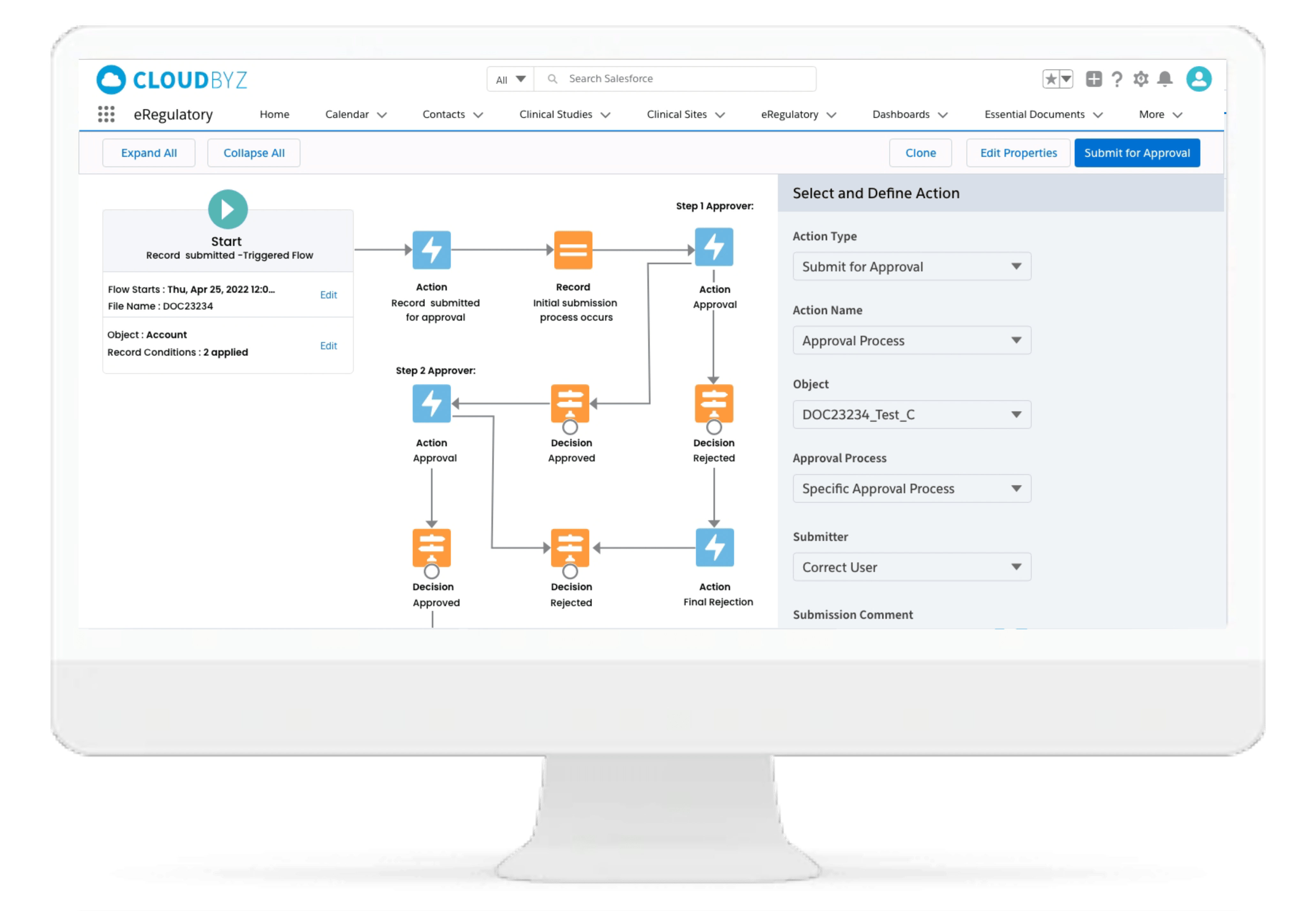
Cloudbyz eRegulatory is built 100% native on the leading Salesforce platform and comes with deep clinical research eSource capabilities.