TRUSTED BY
Cloudbyz eCRF (electronic case report form) is a cloud-based, easy-to-use solution containing various data forms, questionnaires, and study fields designed to capture/collect data in clinical trials and observational studies. This tool enables sponsors and CROs to collect data, digitally, from each participating subject and delivers relatively higher time efficiency as compared to traditional CRF methodology
Our solution, built on the Salesforce platform, enables a customized patient-centric and user-friendly questionnaire format to collect specific data for their clinical hypothesis and research.
Cloudbyz eCRF solution is a pioneer in multi-lingustic data extraction and capture along with gender specific questions.
Other value propositions of the Cloudbyz eCRF solution include a centralized view of captured data with multi-gadget configurability and multimedia content support.
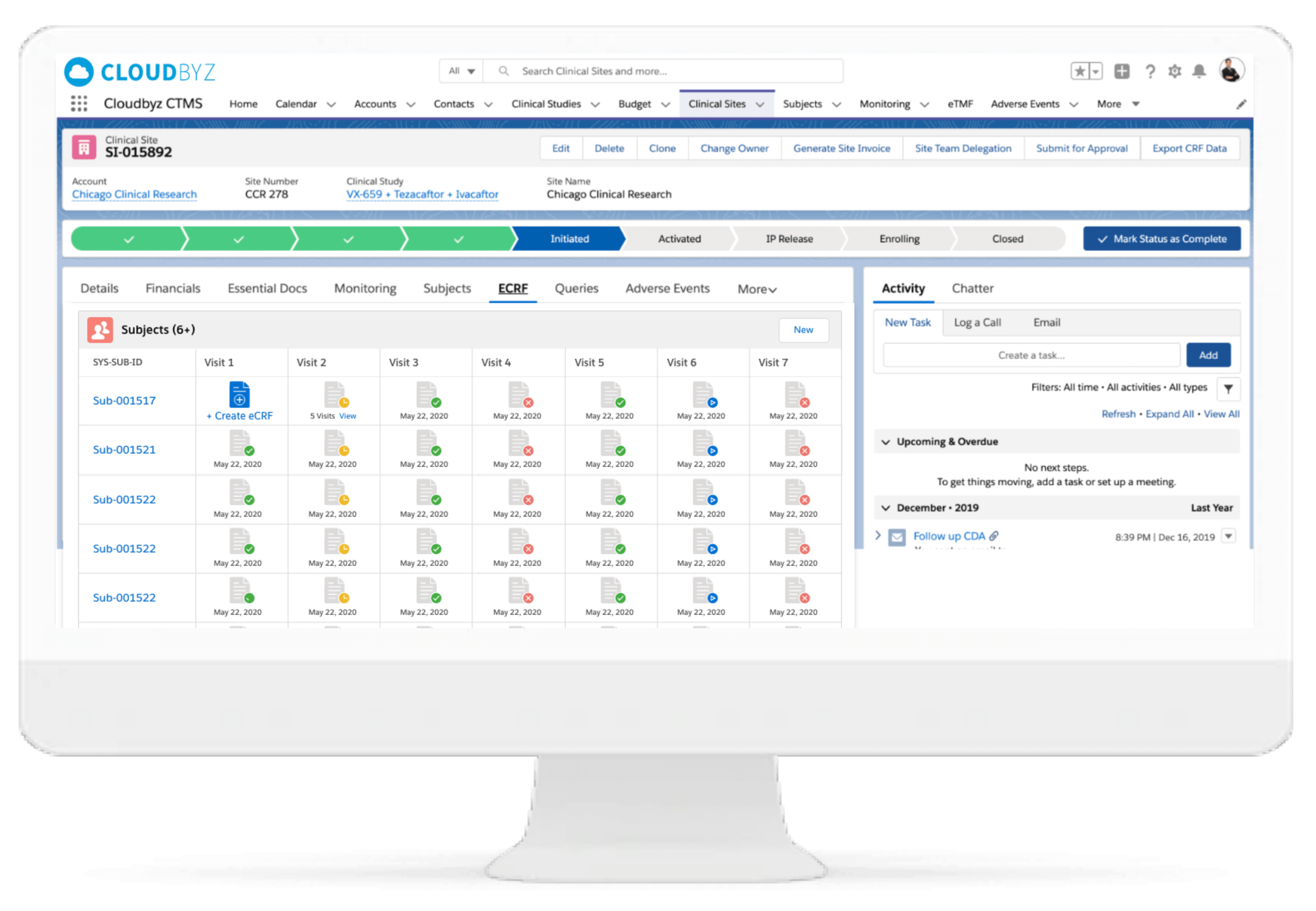
We deliver real-time reporting and centralized data visualization on electronic case report form data. It enables ability to drill-down to review data for quality checks.
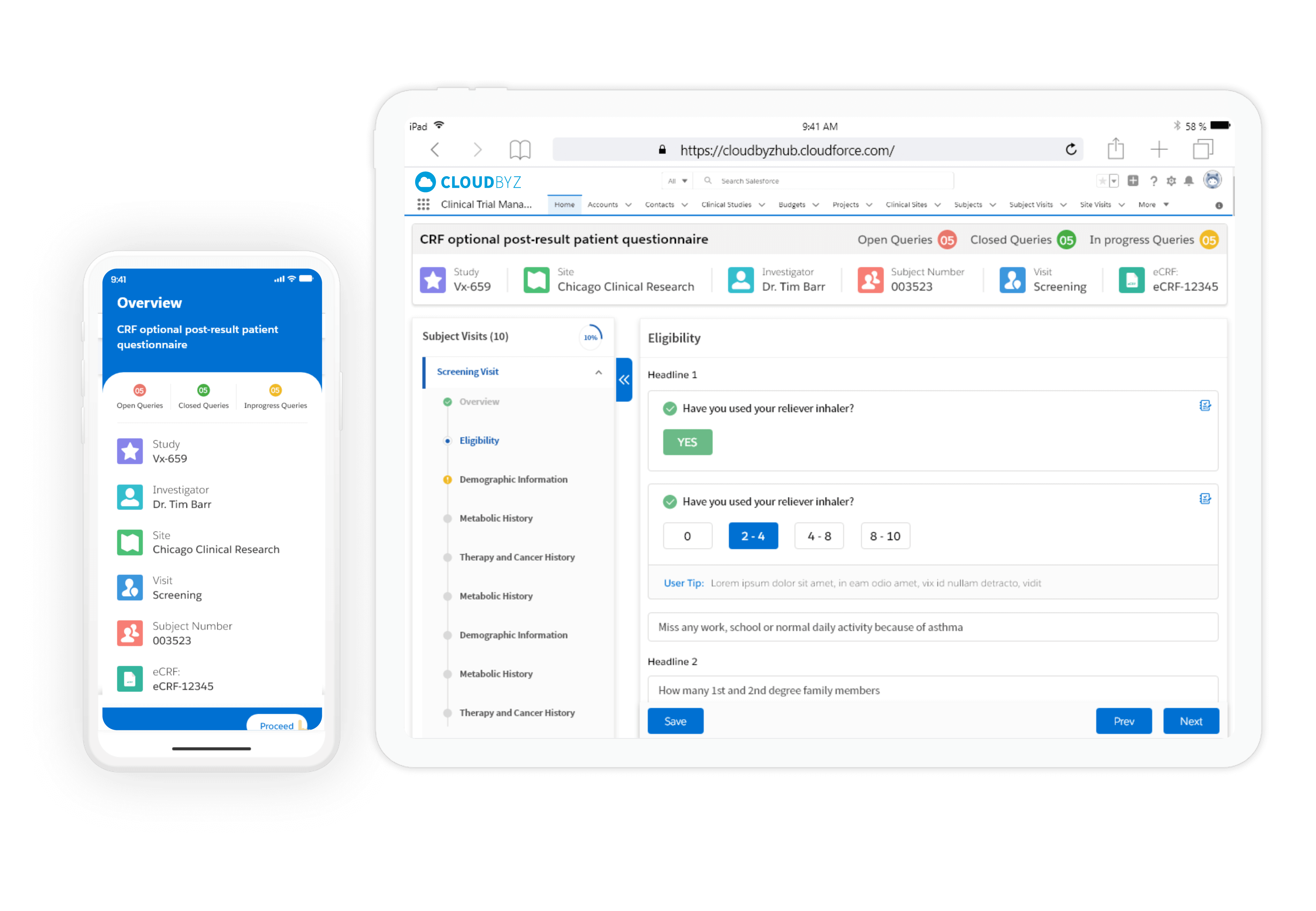
Cloudbyz eCRF software is a cloud based solution wth multigadget configurability. It allows study team to provide their own devices to patients or allow patients to use their own devices.
Cloudbyz eCRF enables electronic transformation of clinical trial case reports for ease use by CRO, sponsors and sites with cloud-based mobile configurability. eCRF/EDC reports reduced data processing and study administration timings for sites and other stakeholders in the clinical trials.