TRUSTED BY
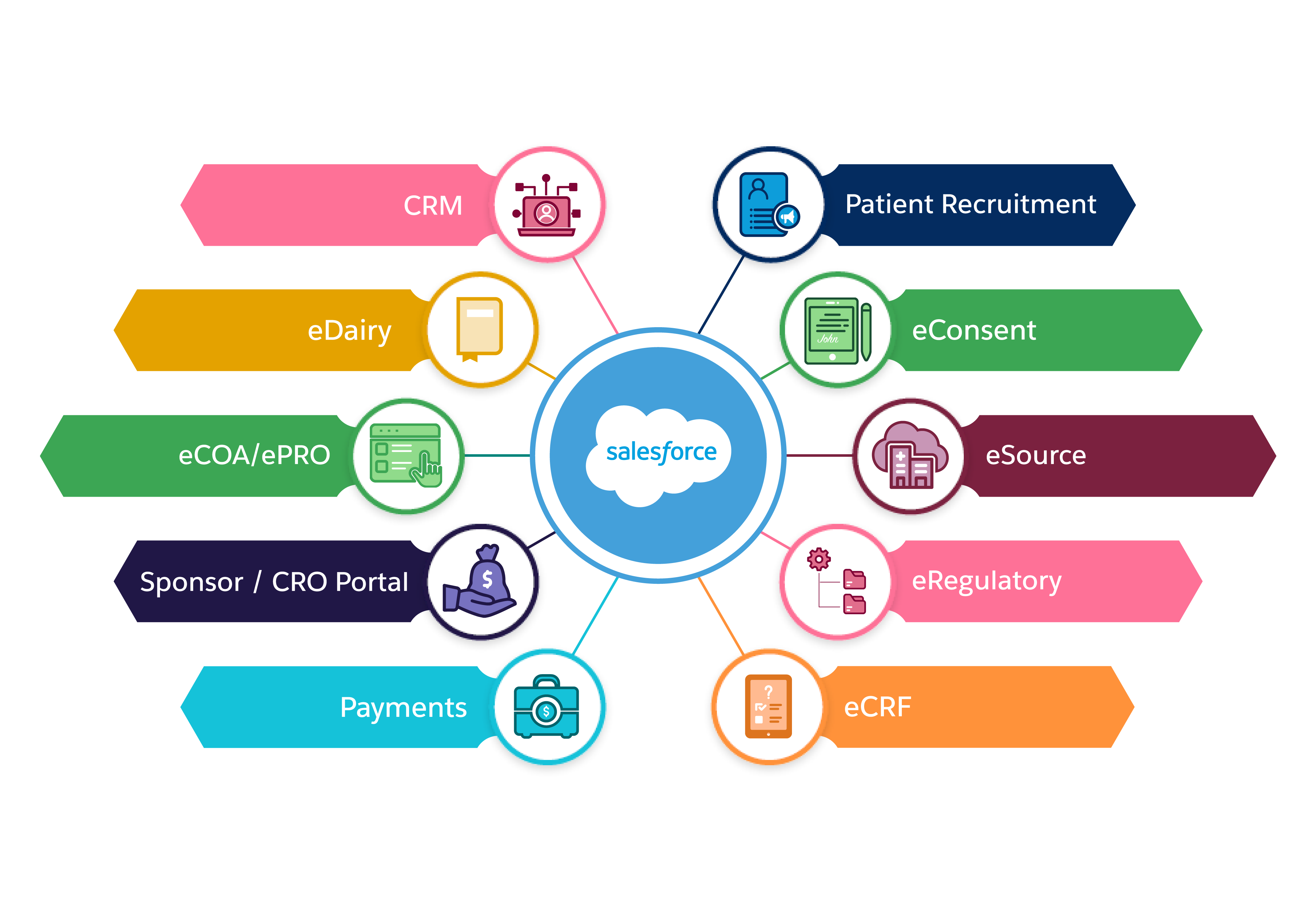
eSource
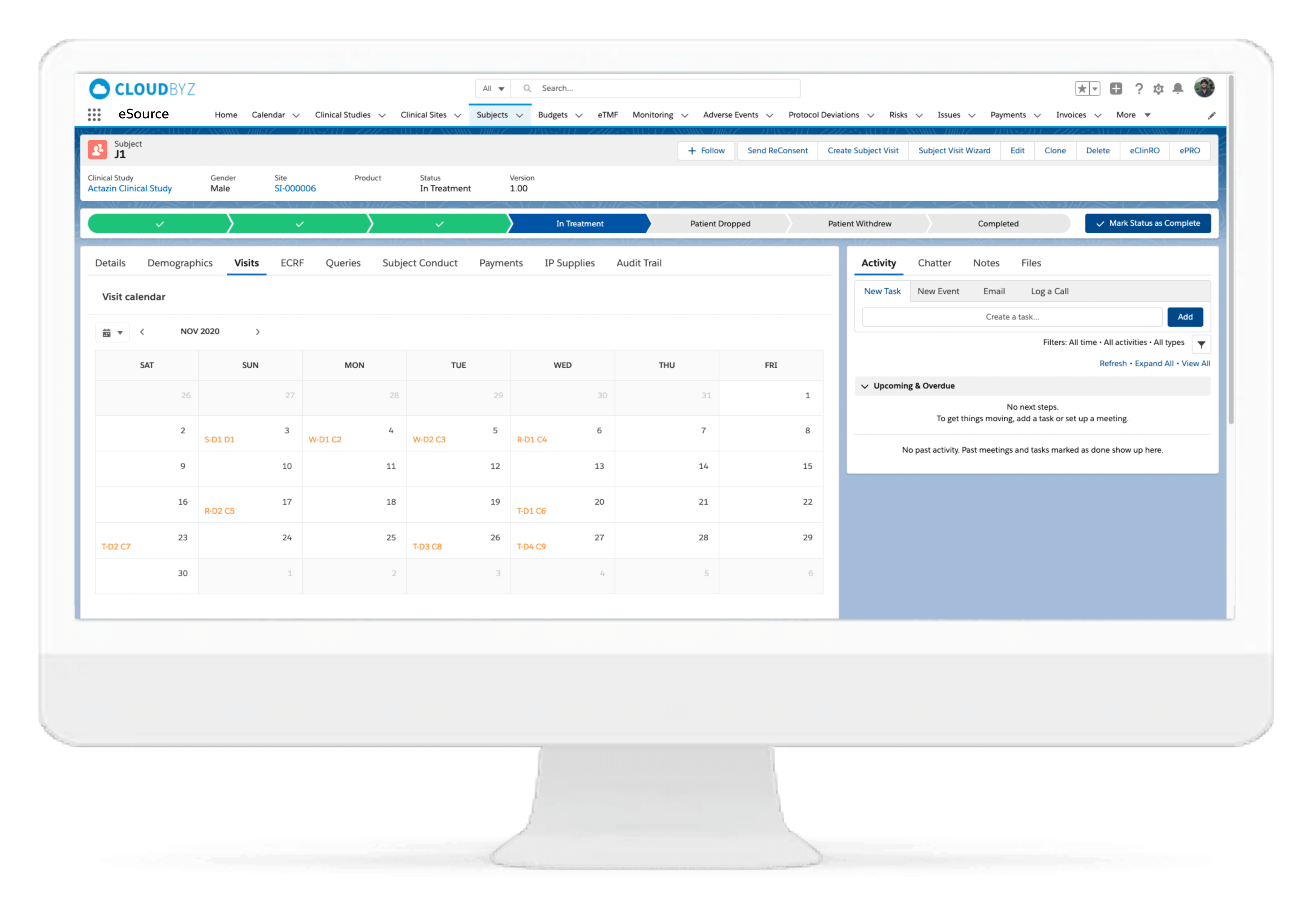
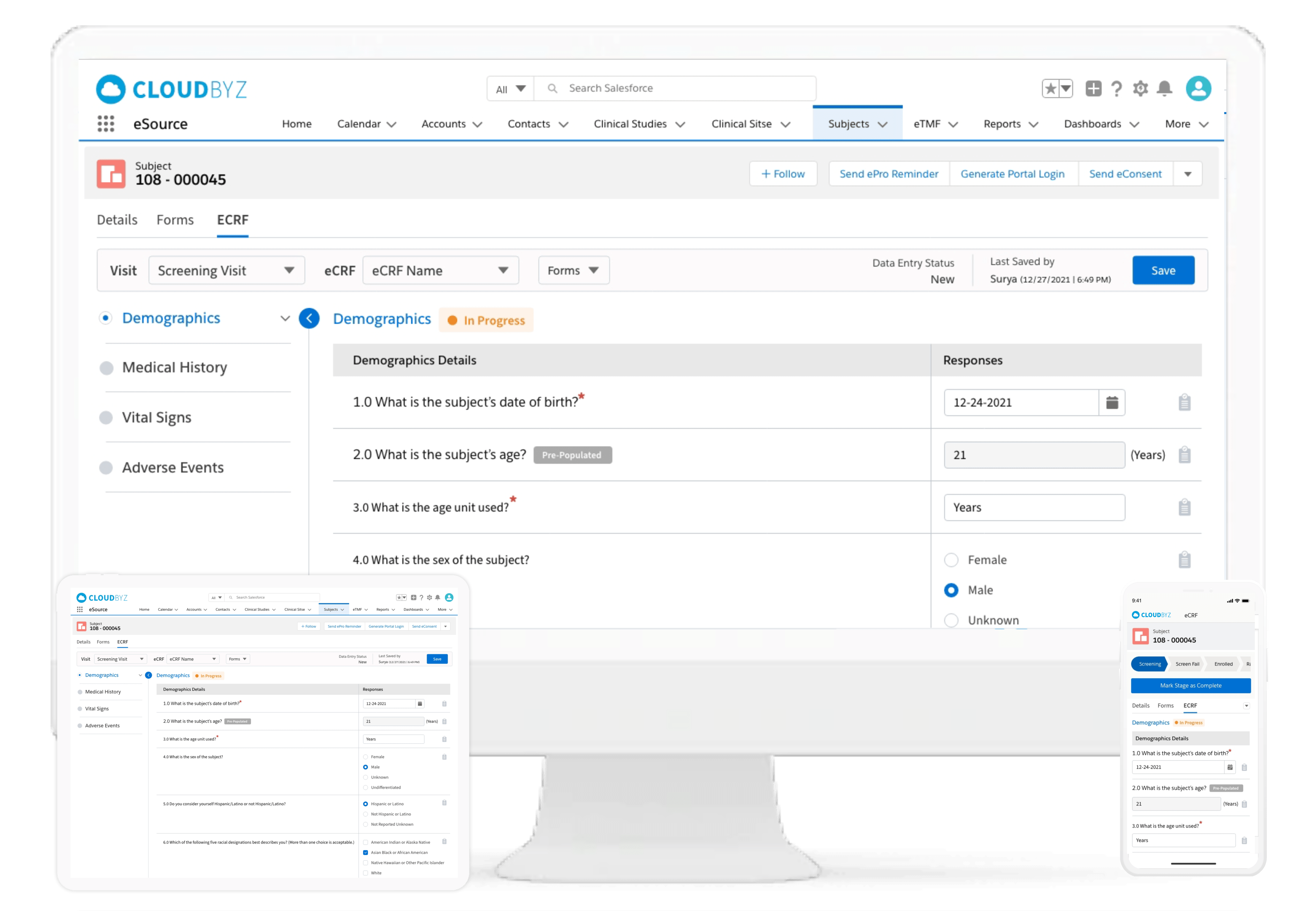
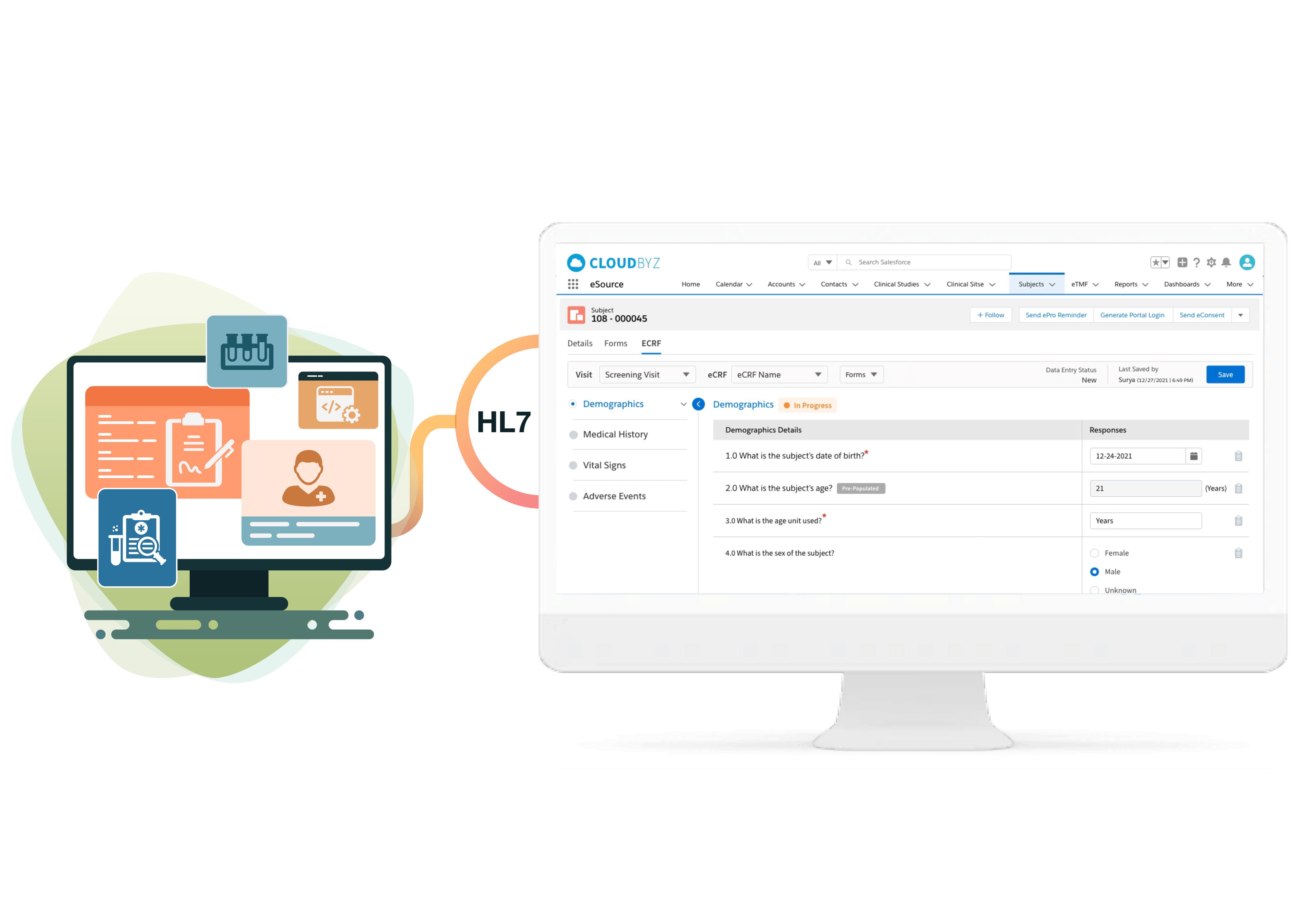
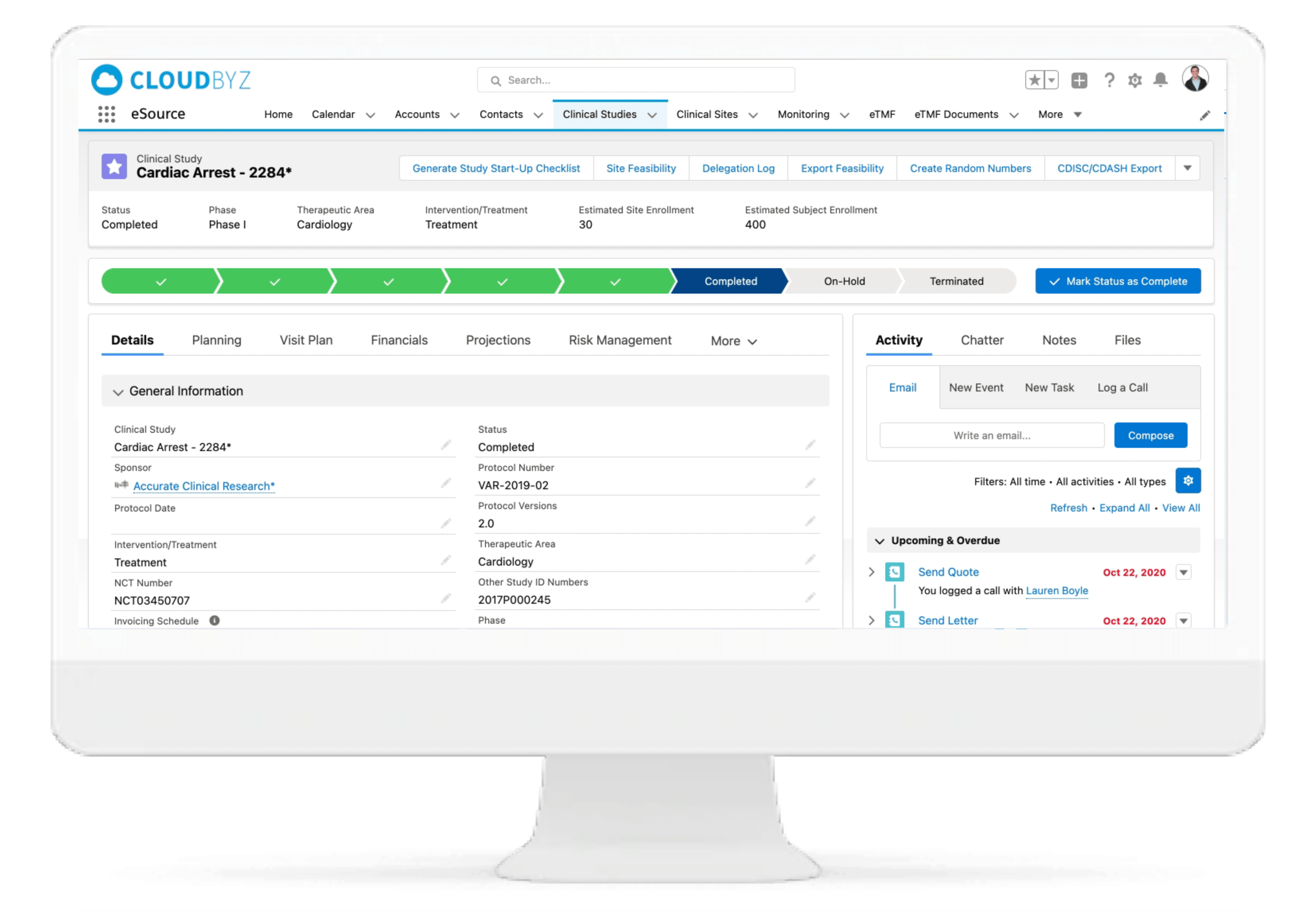
Cloudbyz eSource is a cutting-edge cloud-based solution built to capture clinical data at source and manage clinical data effectively throughout the clinical trial life cycle. Our innovative solution enables clinical research teams to efficiently collect, analyze, and manage clinical data of different complexity and size. Cloudbyz eSource is a scalable solution that meets all essential regulatory compliance requirements such as FDA - 21 CFR Part 11, GCP, GAMP5 and HIPAA.
Using Cloudbyz eSource, customers have eliminated 90% of paper-based processes, improved data quality by 80%, and reported a 37% reduction in overall process.
For each study , the effort spent by site on one patient can be reduced by 8 hours & the time spent in source data verification can be reduced by about 2 hours per patient. Since Cloudbyz eSource is an integrated platform, the solution offers a 65% increase in efficiency in managing overall clinical trial management at site.
Product Features
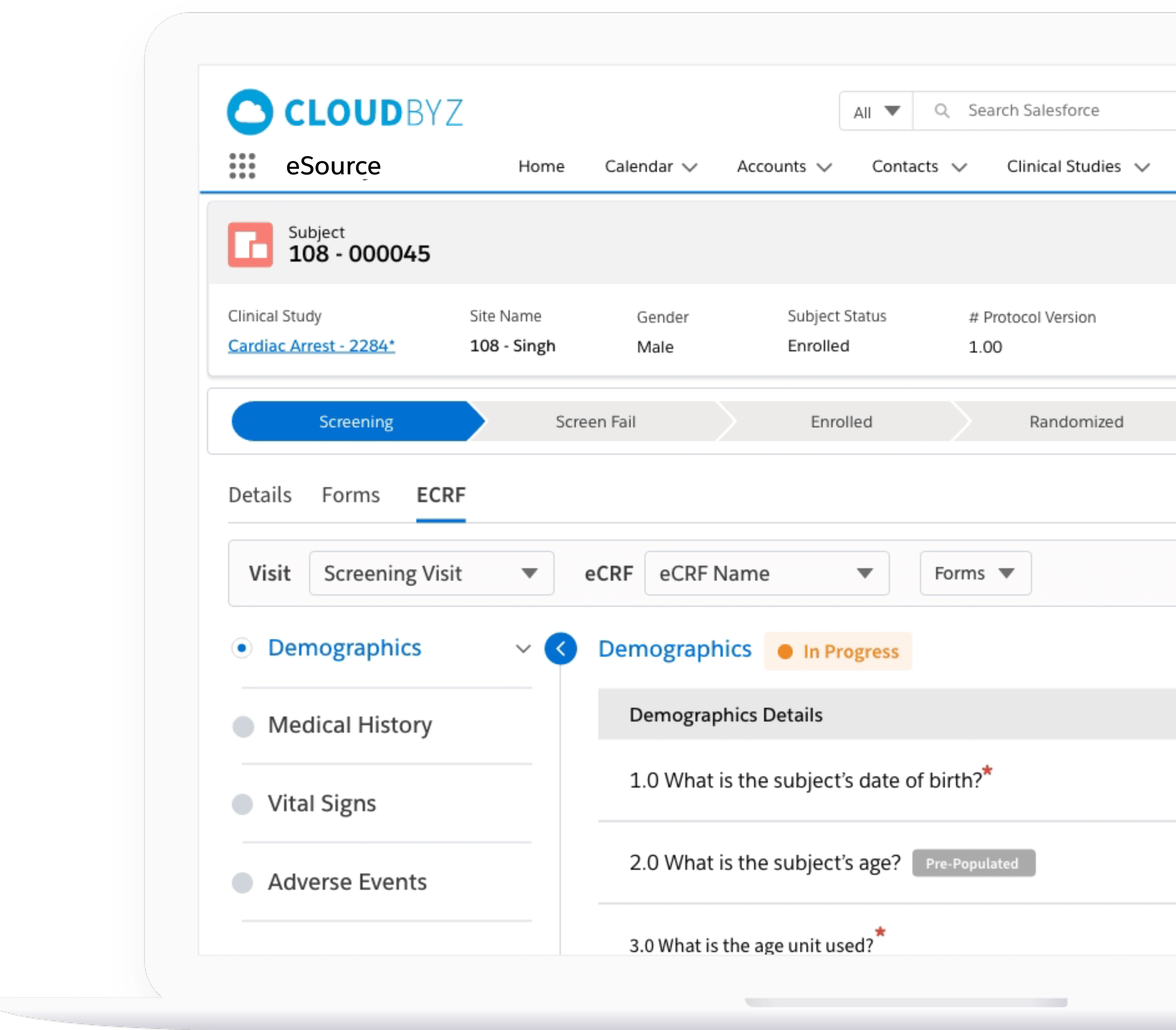
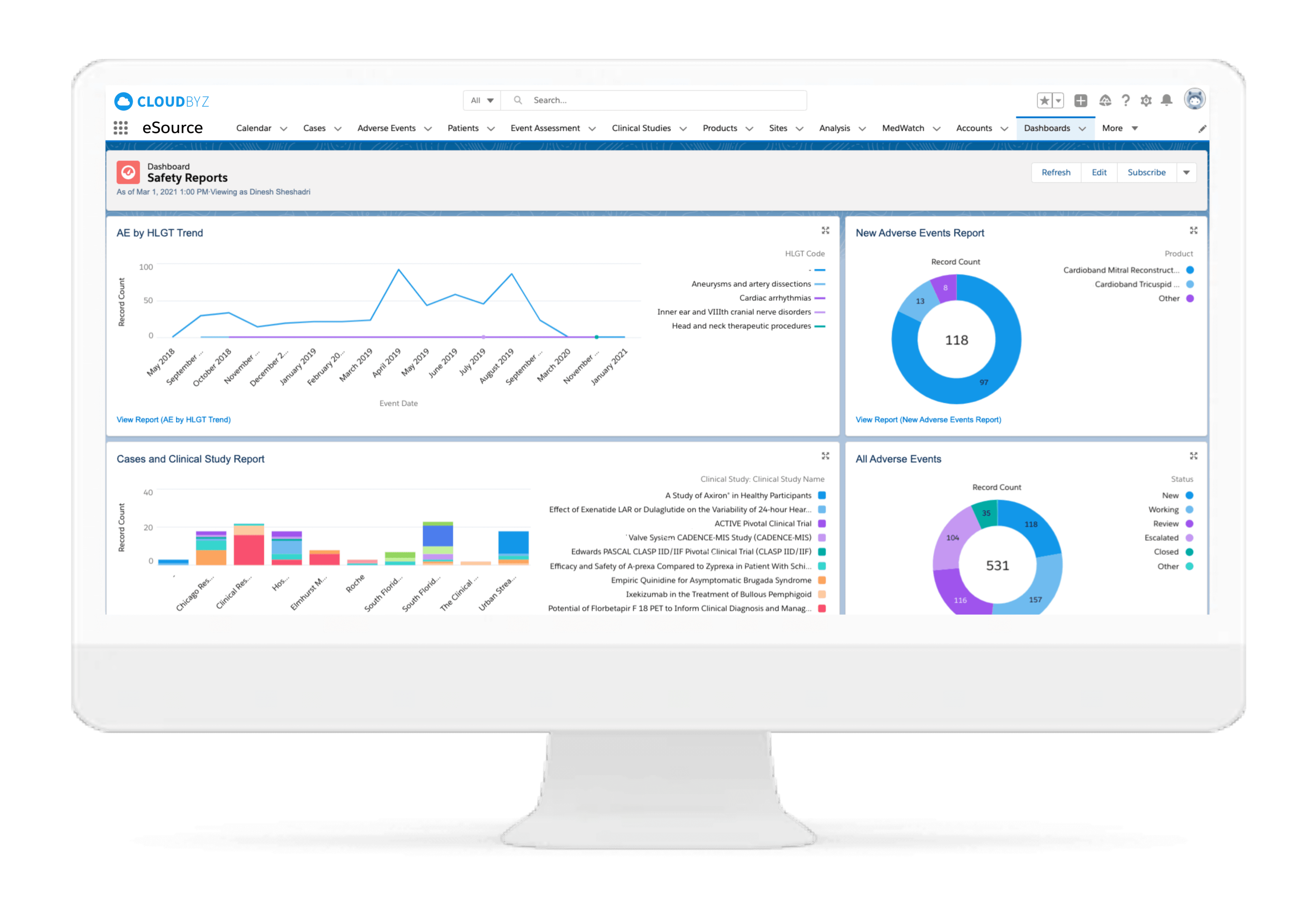
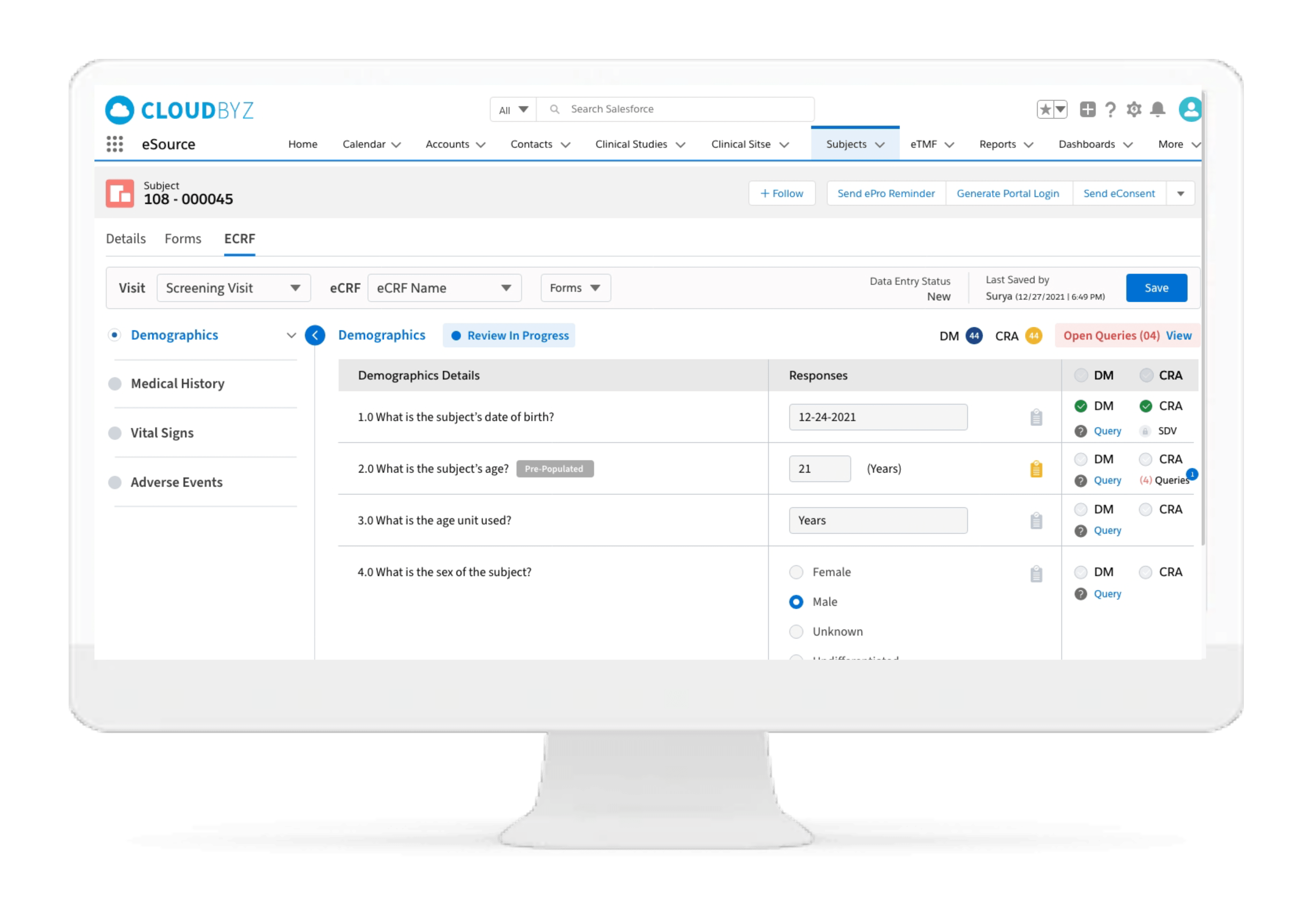
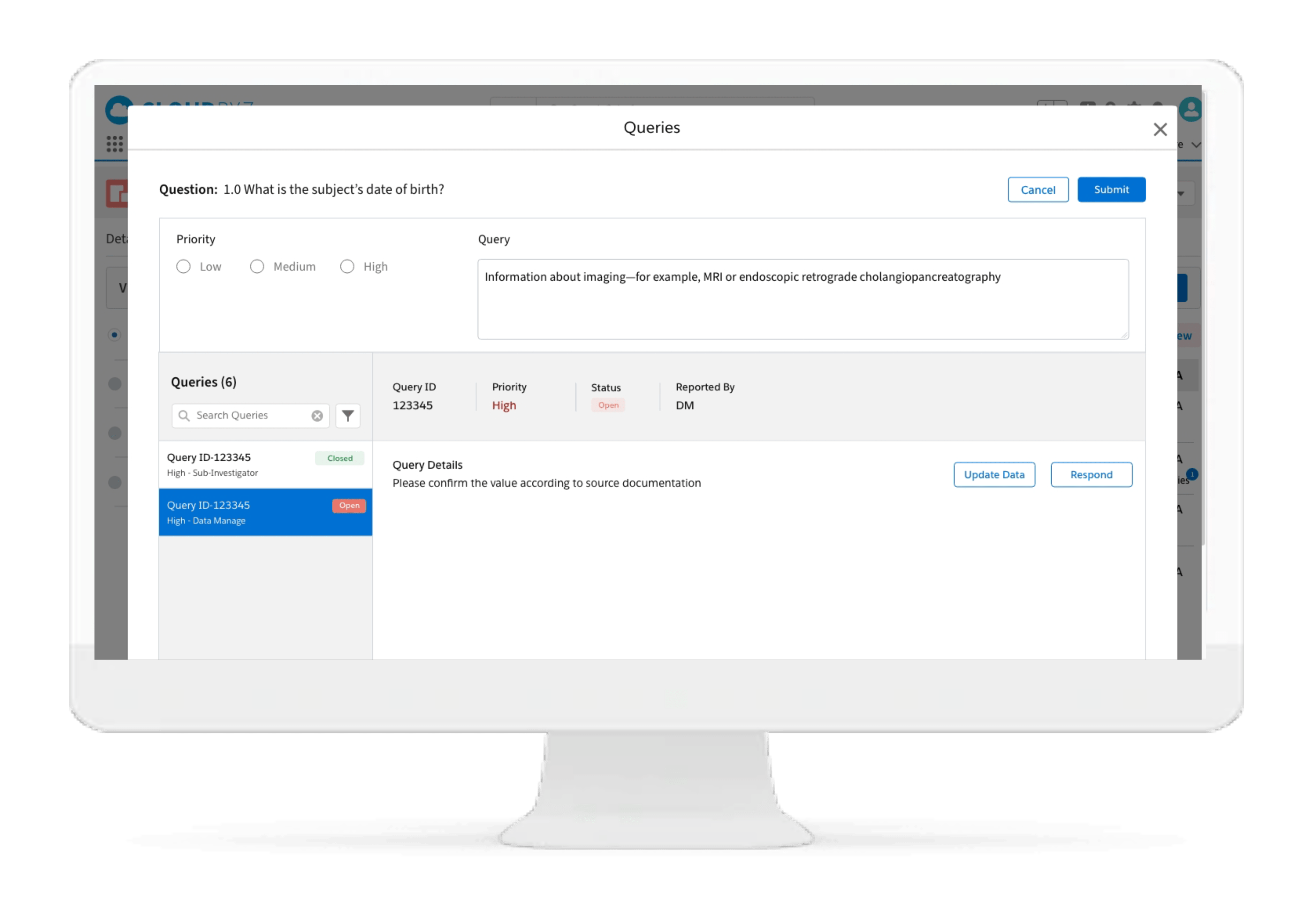
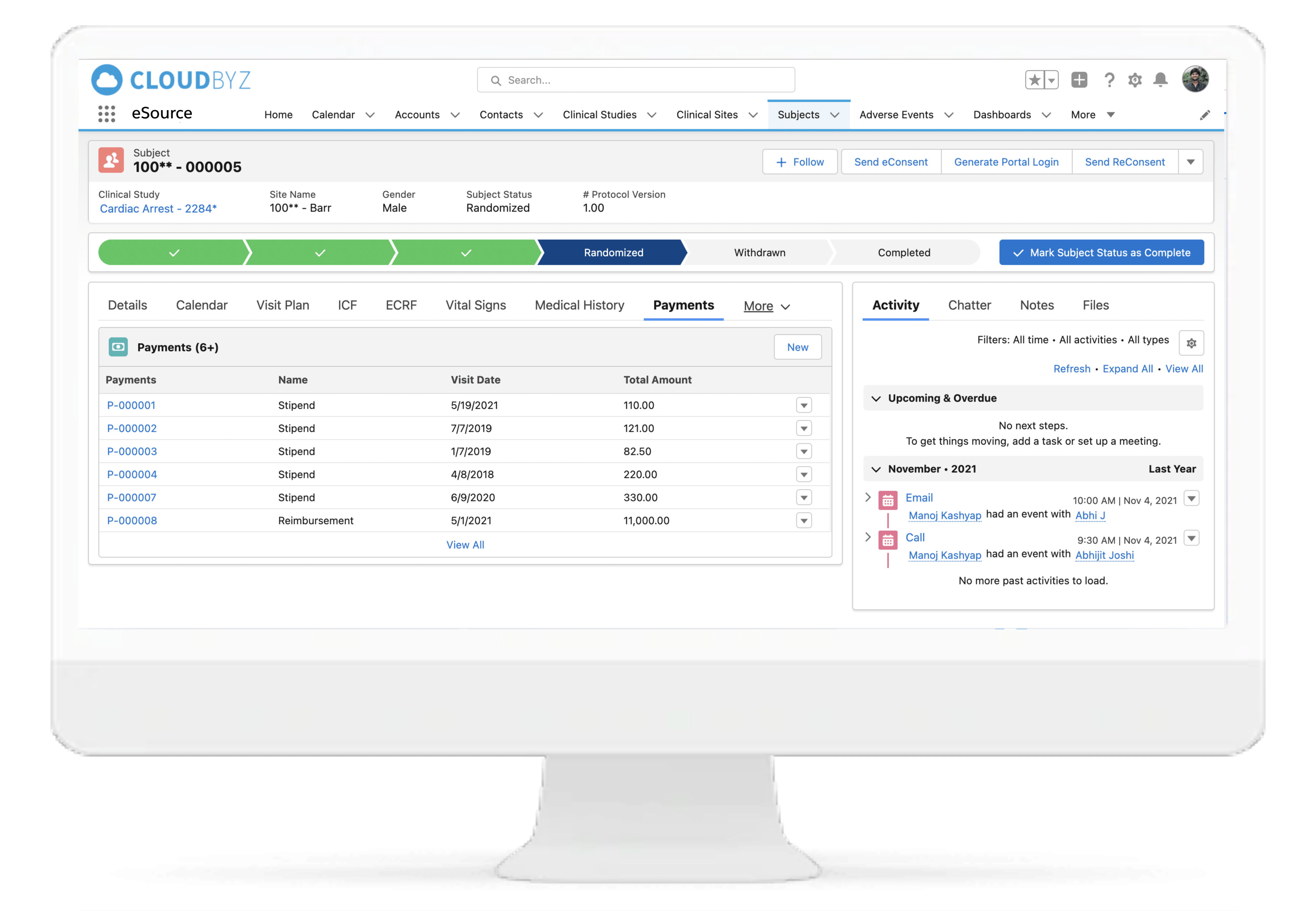
Subject Enrollment Tracking
Subject Enrollment Tracking
The solution tracks the enrollment status of the subjects in a study for the entire duration of the trial. Subjects can be filtered based on their enrollment status to provide insights on whether the study recruitment is proceeding as planned. Build reports and dashboards against this data to provide stakeholders with KPIs to keep studies on track.
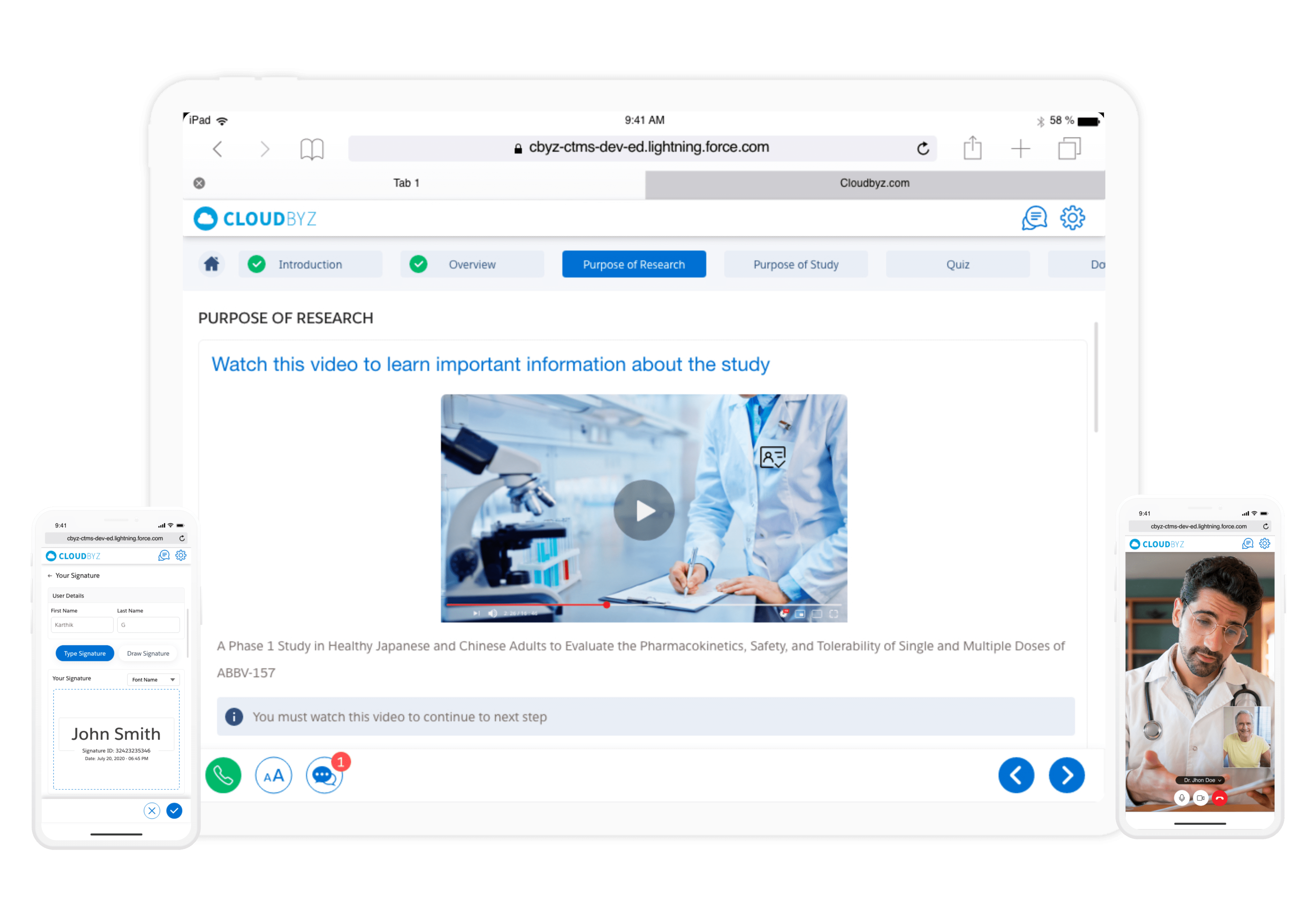
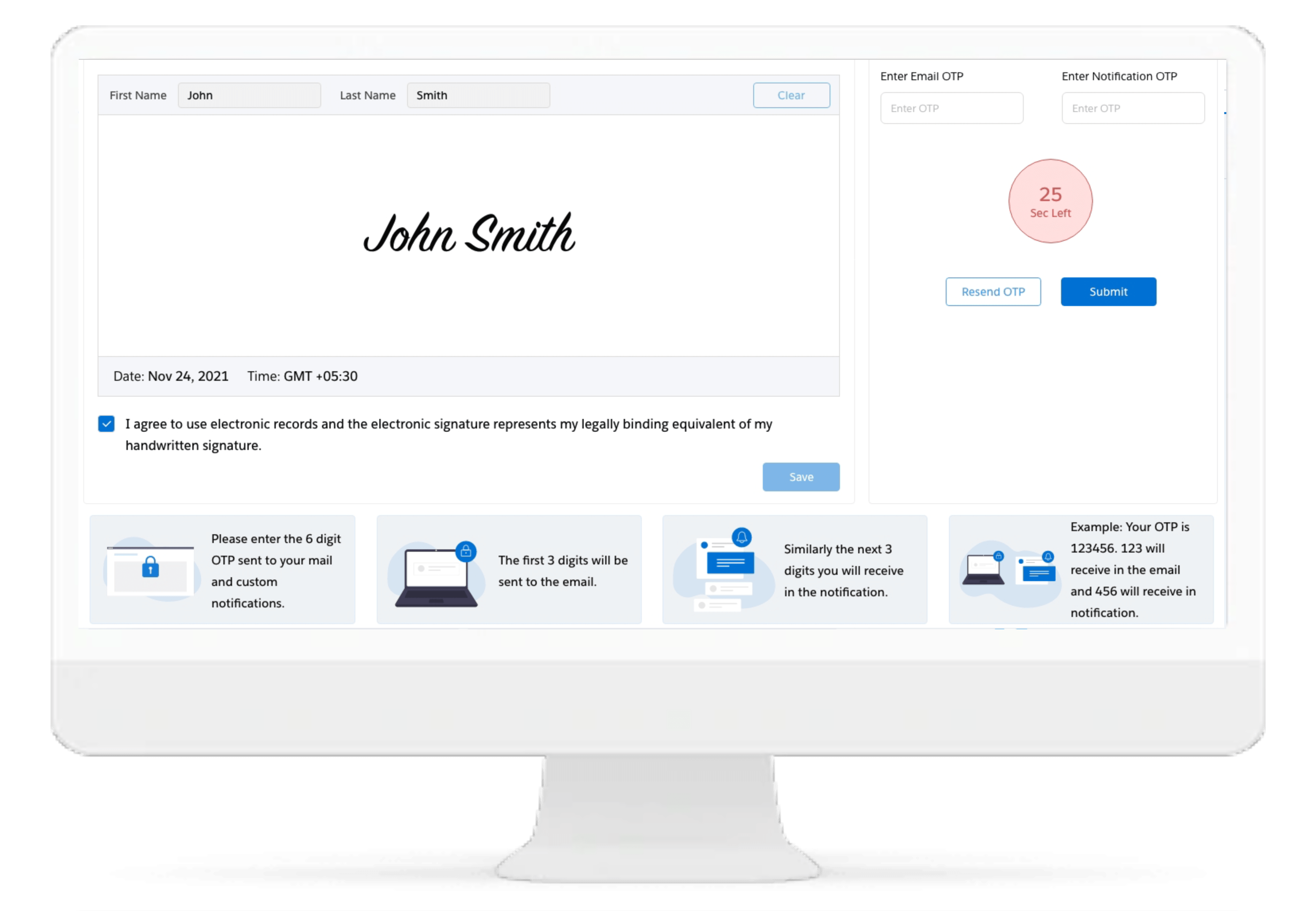
eConsent
eConsent
Cloudbyz eConsent replaces paper-based informed consent documents with interactive, multimedia enabled, and template-driven informed consent on mobile devices. The solution is fully integrated with Cloudbyz eSource enabling real time visibility and central tracking of enrollment metrics across the studies.
Integrated Patient Recruitment
Integrated Patient Recruitment
Cloudbyz eSource comes with an integrated Cloudbyz patient recruitment solution which offers study listing on a public website, patient portal for easy registration, self and pre-screening, appointment scheduling and eConsent and instructions.
Integrated with Safety
Integrated with Safety
Our eSource solutions easily integrate with our centralized cloud-based pharmacovigilance software solution for advanced analytics set-up along with data integrity. It empowers the end-user with proactive pharmacovigilance, smart features with data-backed predictability, scalability and cost-effective support.
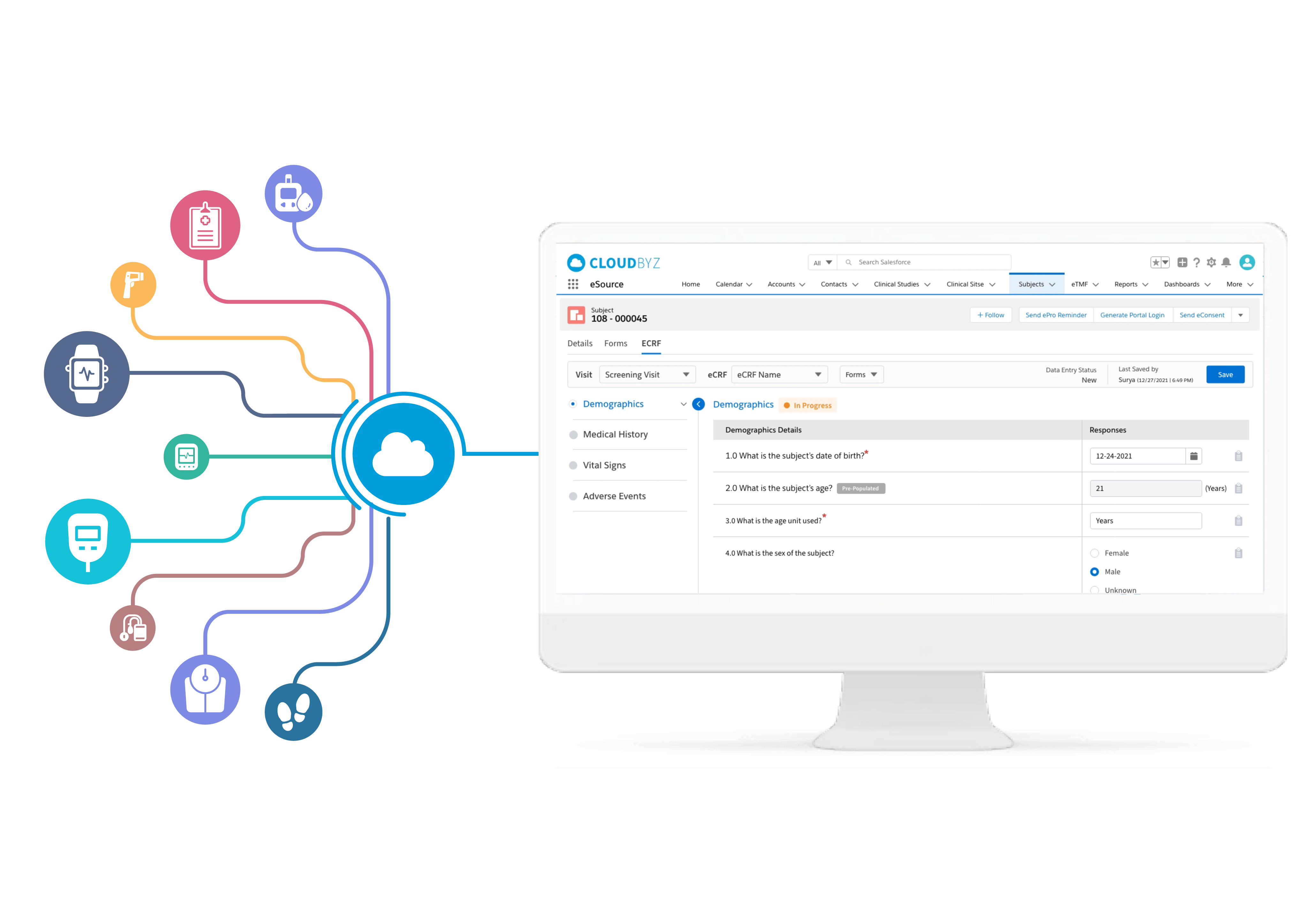
eCOA, ePRO & eDairy
eCOA, ePRO & eDairy
The Cloudbyz eCOA solution replaces conventional paper-based, patient-reported outcomes by leveraging innovative technologies to capture clinical outcome data. This includes ePROs, ClinROs and eDiary functionalities. Our solution is mobile friendly, supports time-sensitive data collection, enables upload of multimedia content for better patient education, and uses various edit checks to minimize data errors.
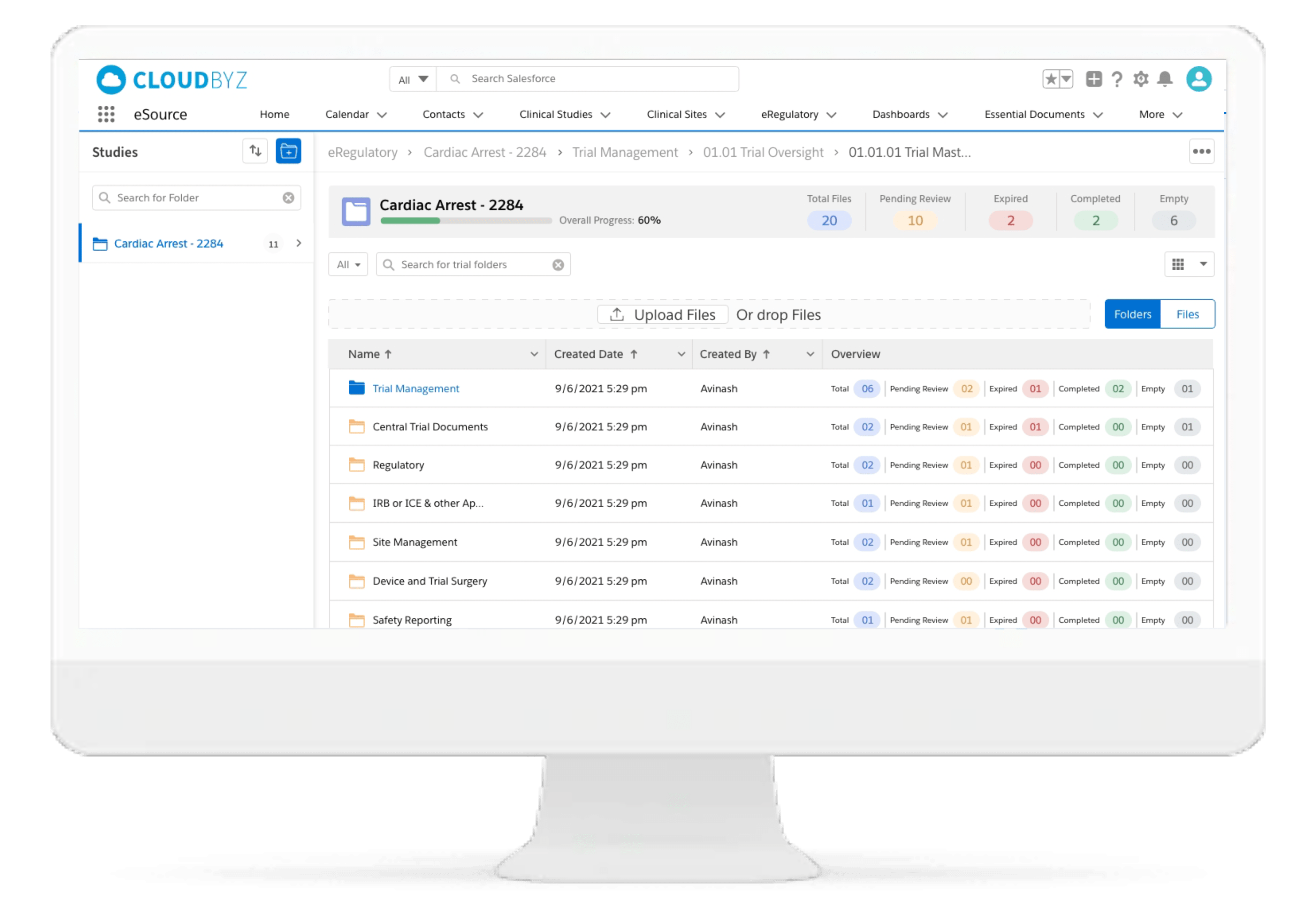
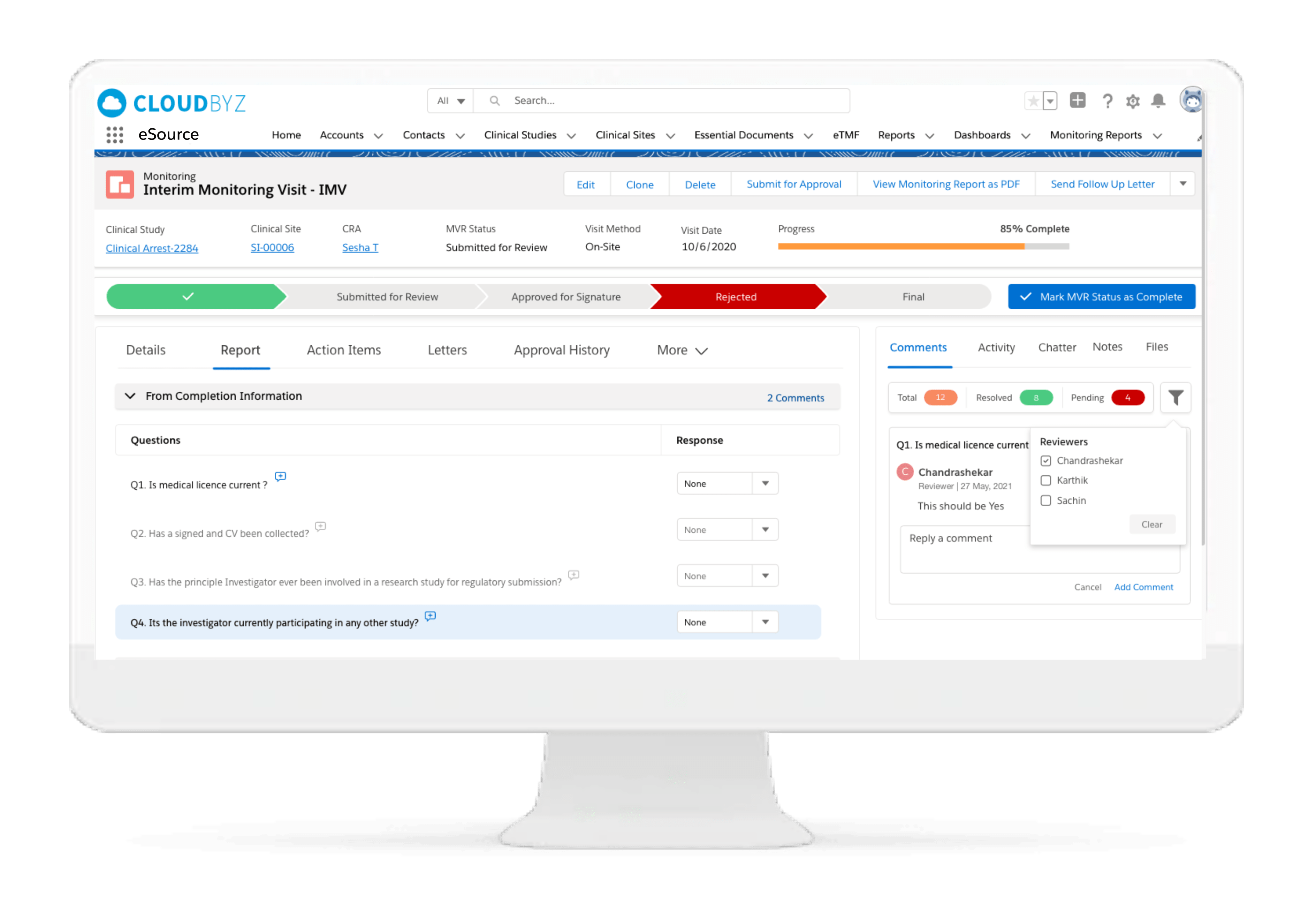
eRegulatory
eRegulatory
Cloudbyz eSource comes with an integrated eRegulatory solution complete with a configurable site binder structure supporting both industry standard eISF or custom binder structure. Clinical trial documents can be saved directly into eRegulatory upon review and approval.
With integrated sponsor and CRO portals, sponsors and CROs can directly access regulatory documents from the eRegulatory solution and transfer to their respective eTMFs, as applicable.
Key Benefits
Cloudbyz eSource is built 100% native on the leading Salesforce platform and comes with deep clinical research eSource capabilities.