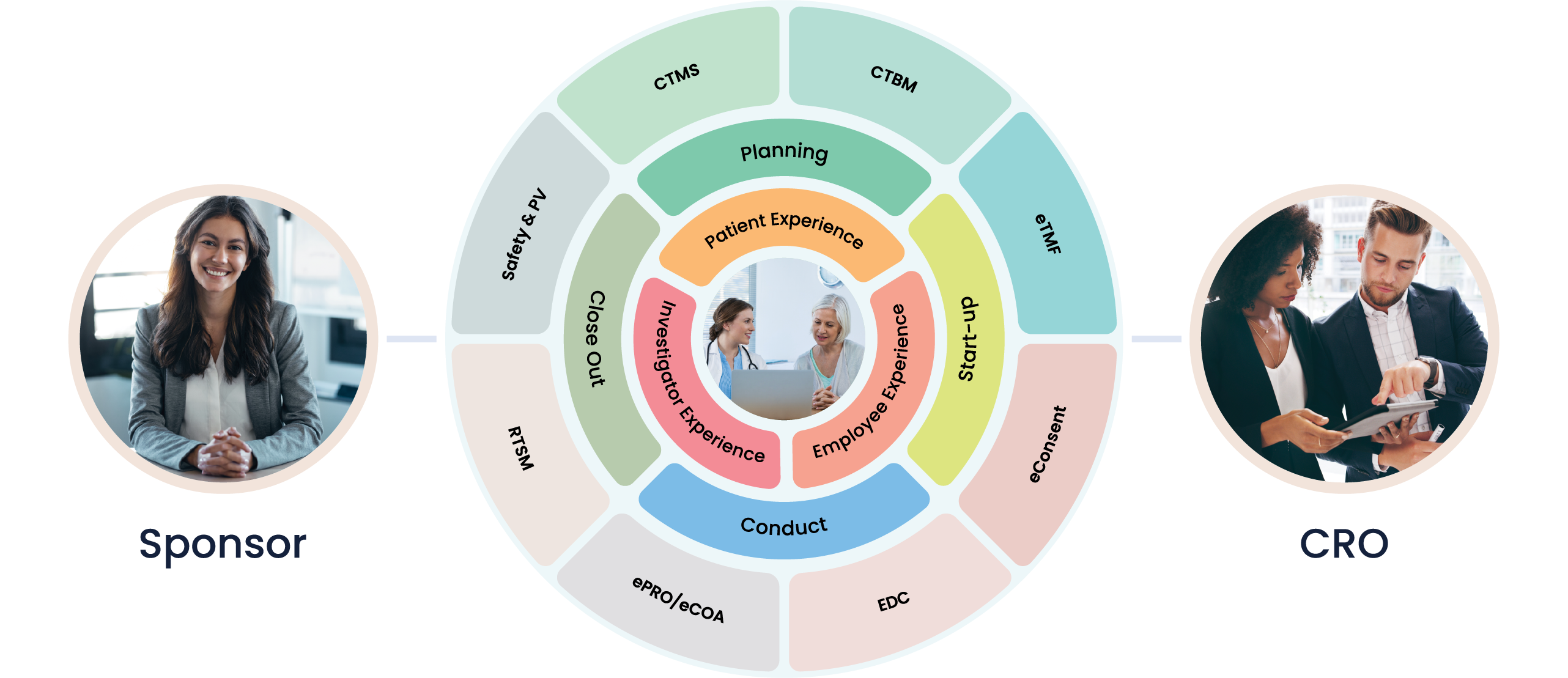
At Cloudbyz, we understand the pivotal role that clinical trials play in the Vaccines, Blood & Biologics industry. Recognizing the distinct complexities and regulatory nuances that come with these processes, our solutions are designed to simplify these tasks, enhancing efficiency and delivering accurate, actionable results.