TRUSTED BY
eConsent
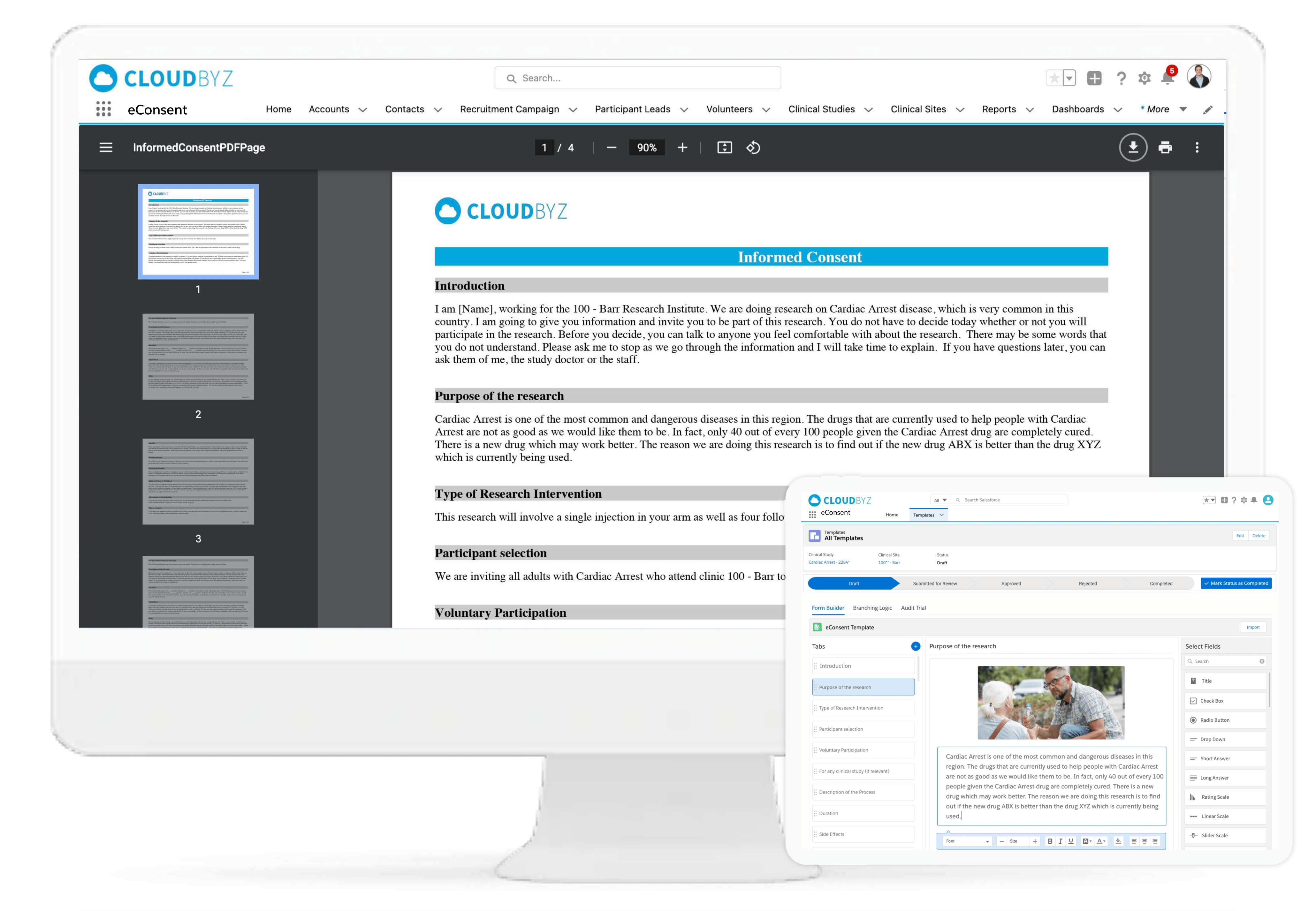
Cloudbyz eConsent replaces the paper-based informed consent document with interactive, multimedia-enabled, and template-driven informed consent on mobile devices. The Cloudbyz eConsent solution includes interactive multi-media approaches for the presentation of information and feedback mechanisms to test and reinforce participant comprehension.
Built on the powerful Salesforce platform, our solution supports a unique set of features that increase the efficiency of study teams, provide real-time visibility and collaboration to sponsors, and enhance the patient experience.
Product Features
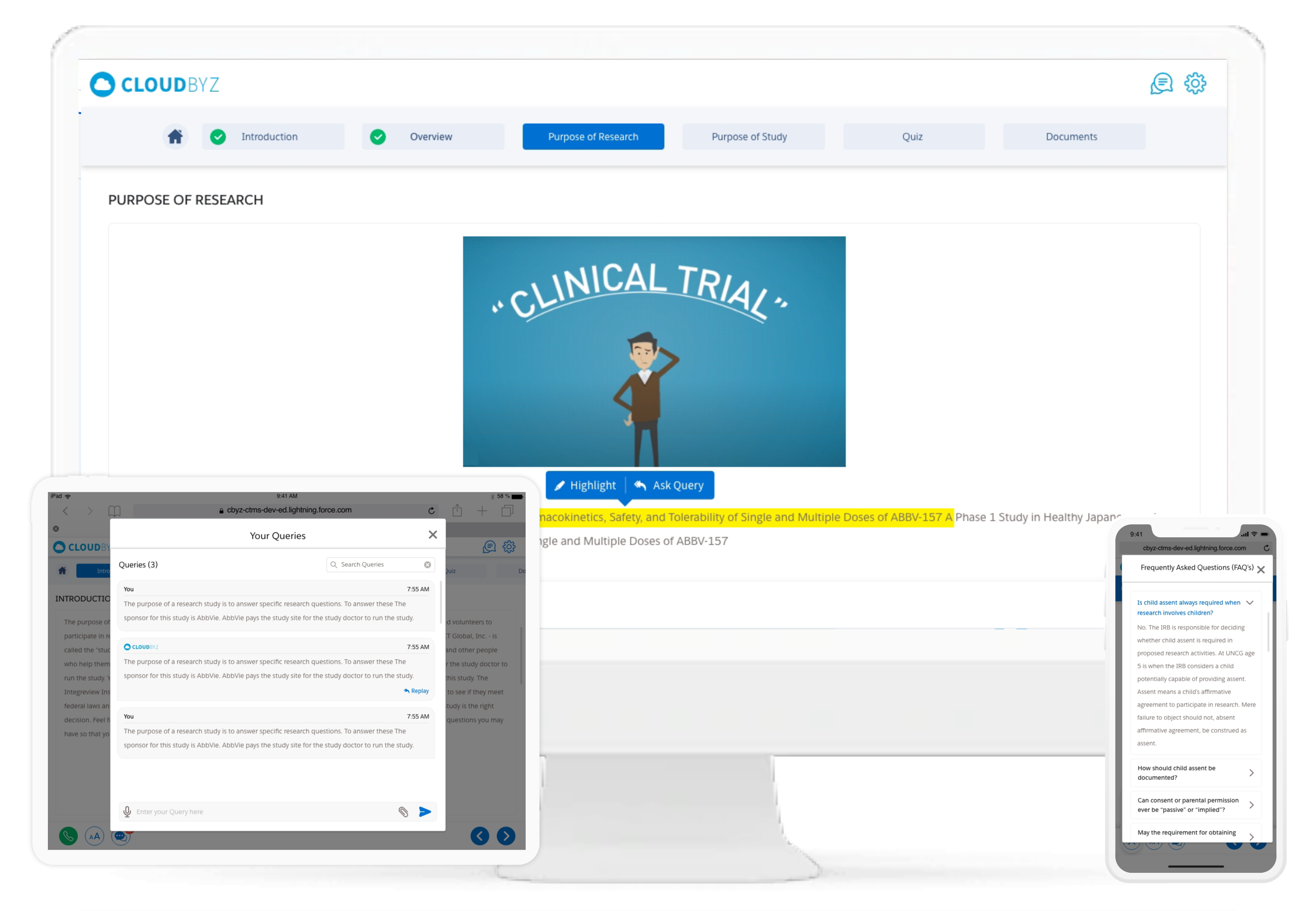
Participant Engagement
Participant Engagement
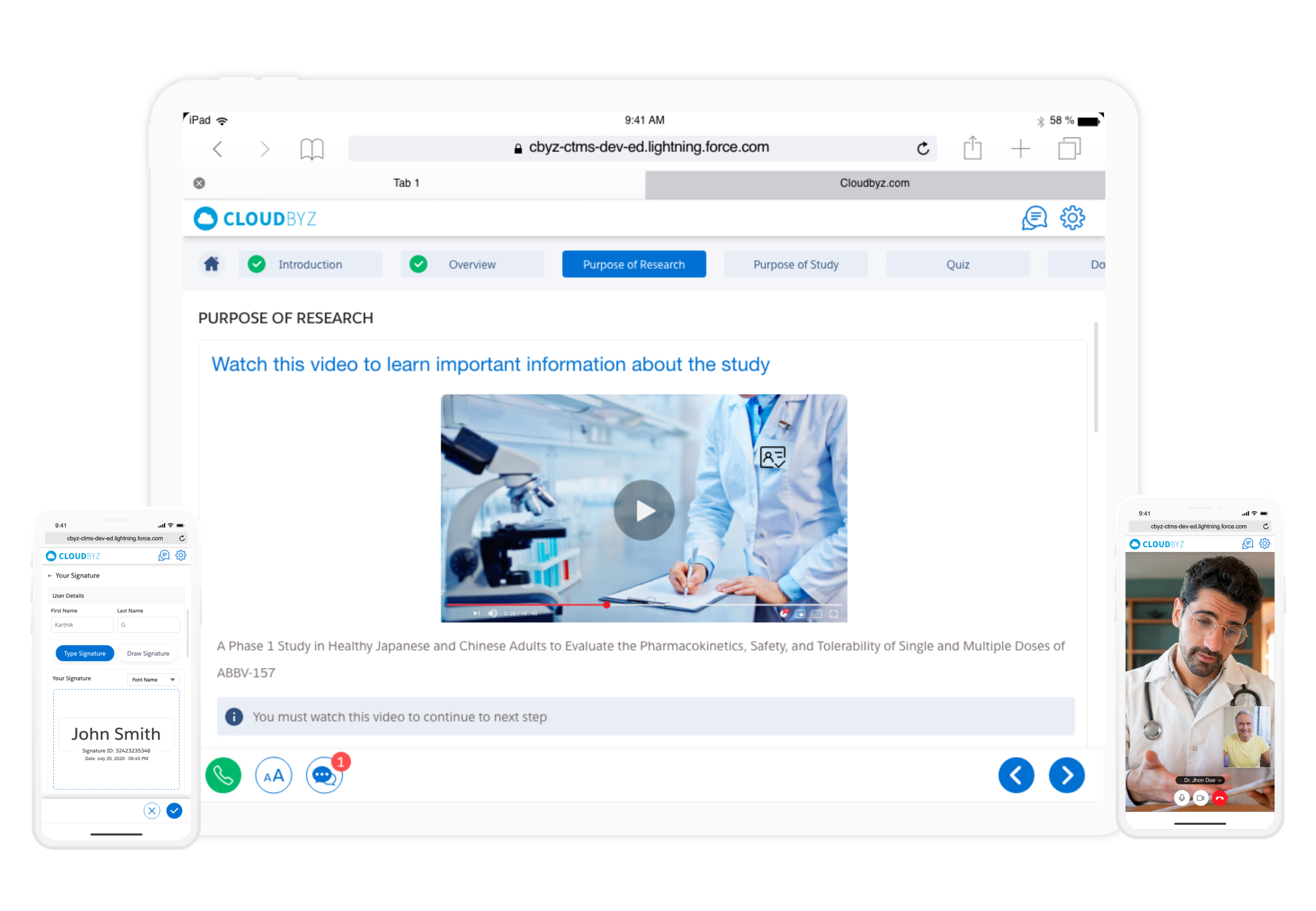
Boost participant engagement with a question-and-answer-based consenting process. Participants can submit their questions about the trial directly to the study team through chat, or by highlighting text that they are unsure about. Threads are automatically saved for future reference.
Participant Compliance
Participant Compliance
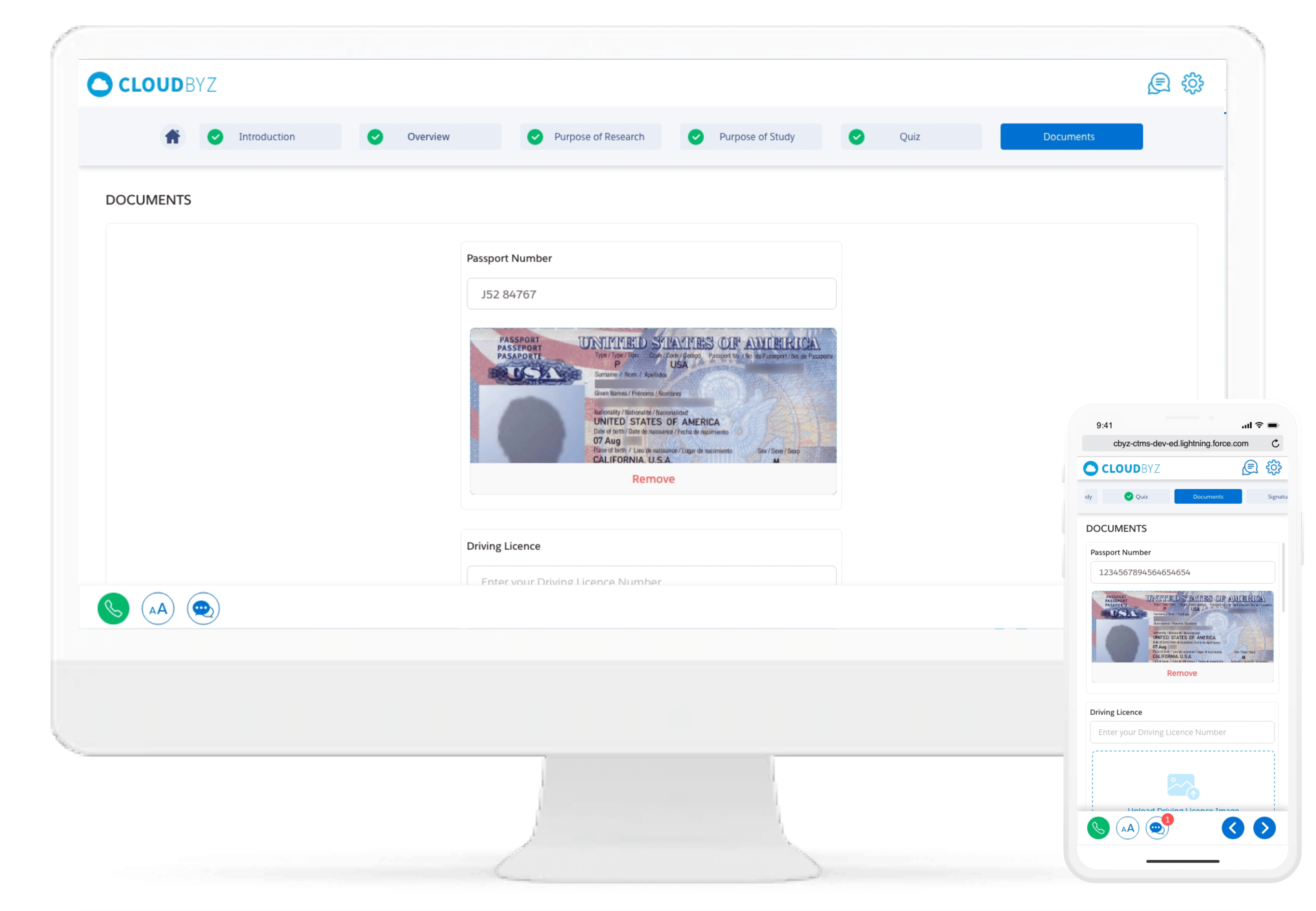
Decrease drop-out rate and increase compliance with a superior and interactive user interface. Participants can review the consent forms and documents on their electronic or mobile devices without feeling rushed. Reduce patient visits - participants can complete the consent process from the comfort of their homes.
eConsent Participant Quiz
eConsent Participant Quiz
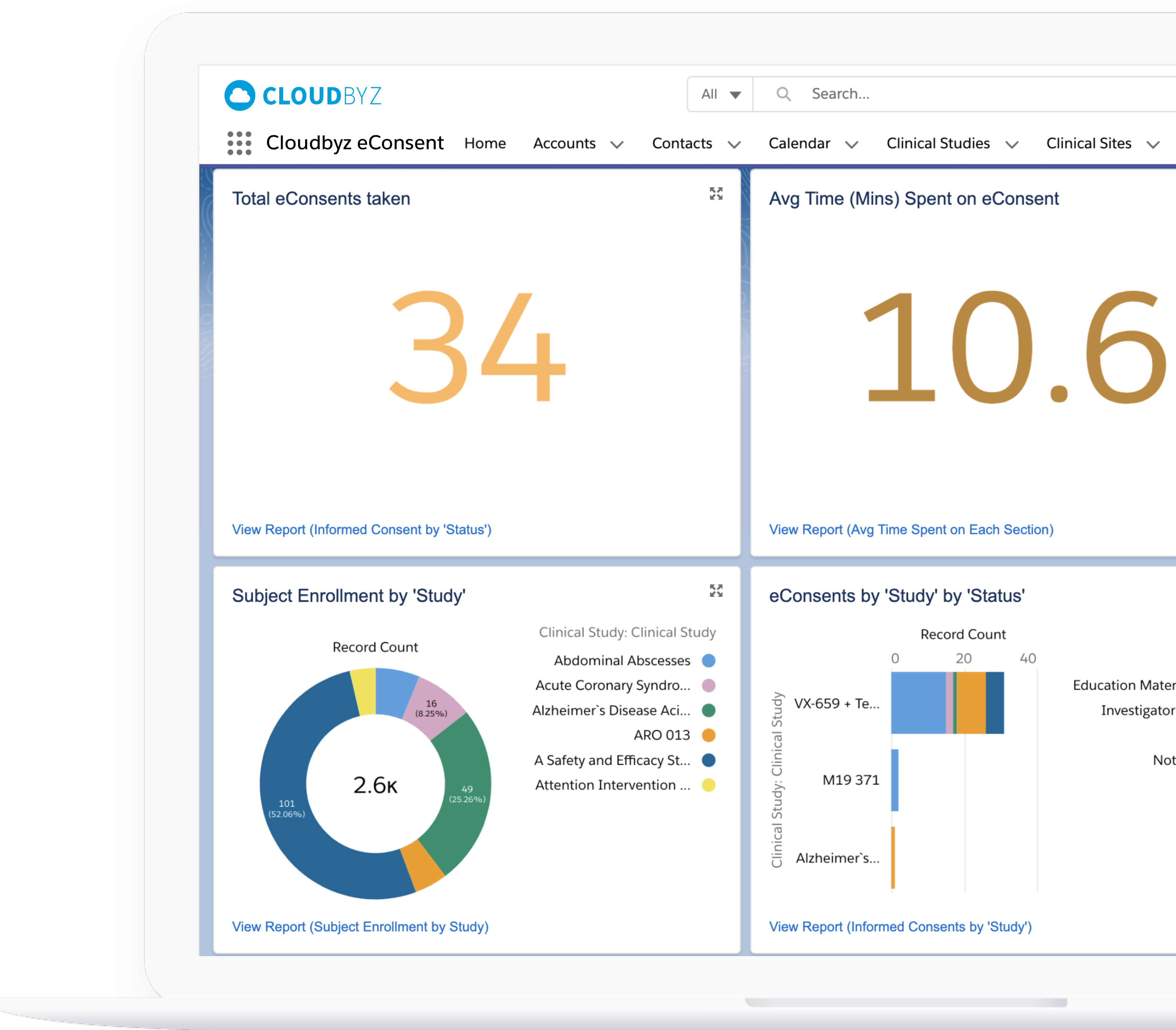
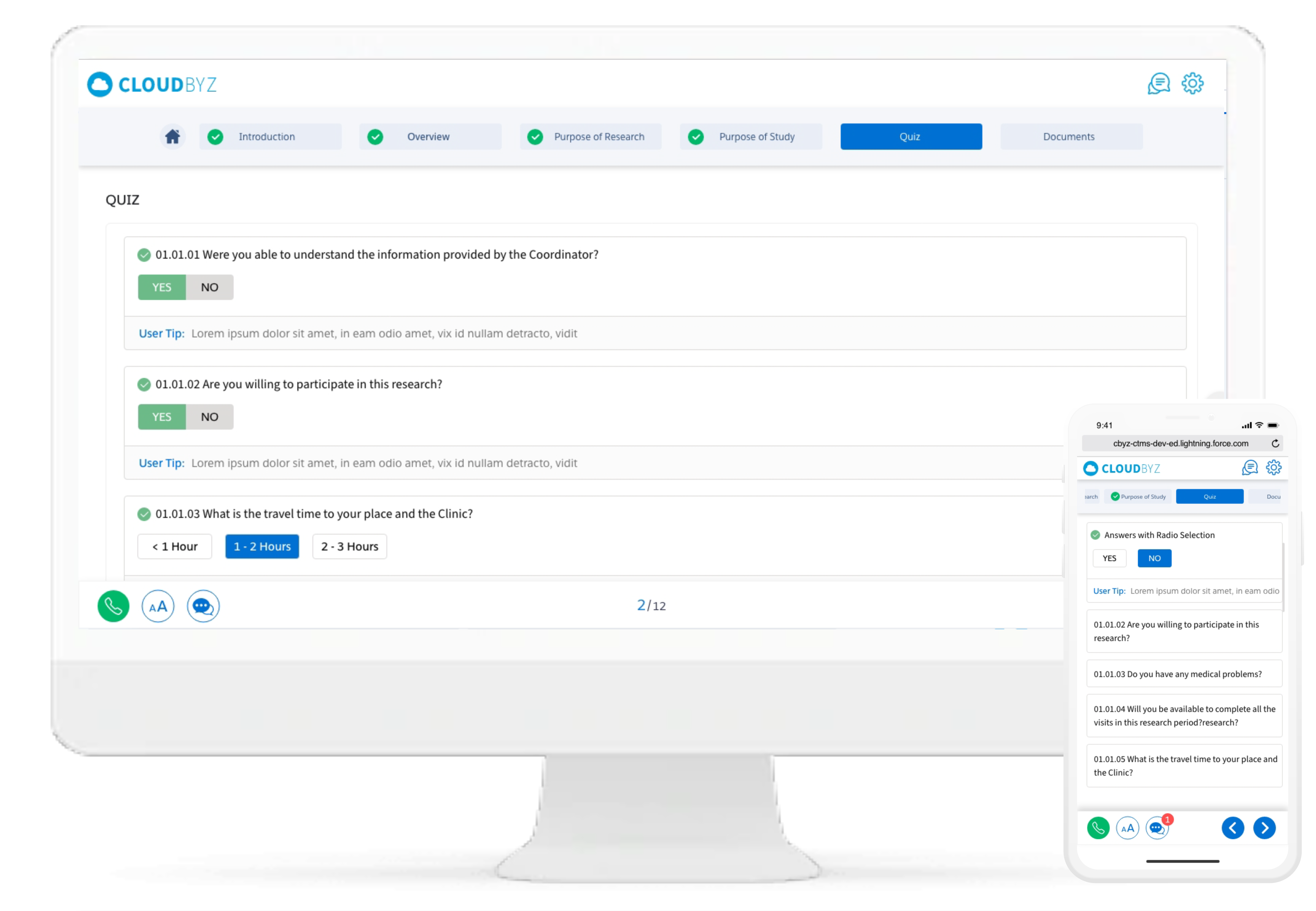
Include quizzes for participants at various stages of the consent process to ensure understanding and comprehension. All responses are tracked, enabling study teams to make changes to questions if a consent quiz is difficult to understand. Track the progress of the consent process through various metrics and analytics.
Informed Consent Procedure Compliance
Informed Consent Procedure Compliance
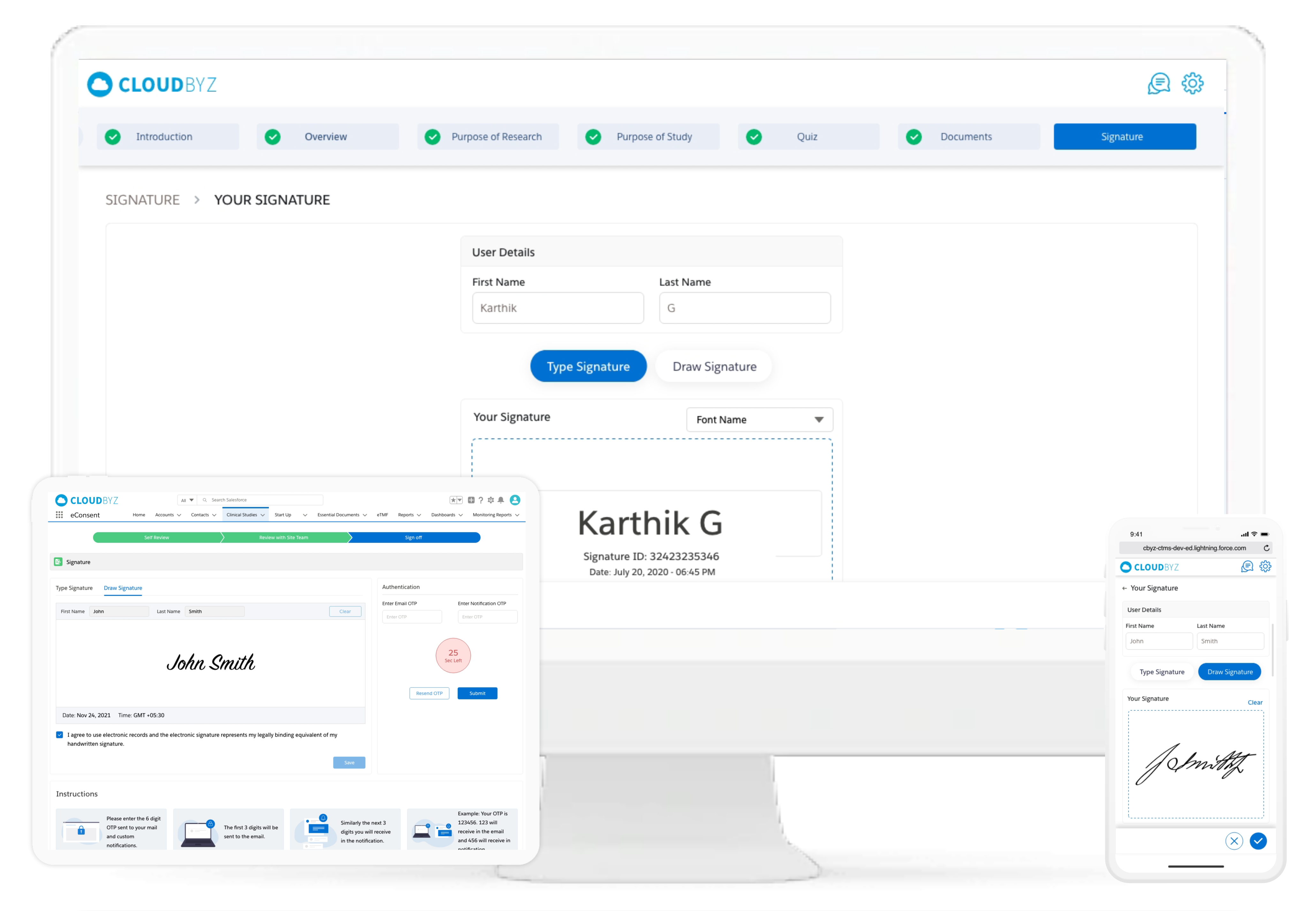
Improve compliance to the consent procedure by standardizing the electronic signatures with various checks and controls. Control electronic signature workflows for the participants and the study staff. The system is HIPAA and 21 CFR part 11 compliant, with features like Audit Trail and Version Control.