clinDICOM AI
Welcome to our comprehensive DICOM data extraction and redaction solution that empowers healthcare organizations to effectively manage sensitive patient information. With a wide range of features, our system ensures accurate and secure handling of DICOM files while adhering to regulatory requirements and maintaining user-friendly experience.
Experience the power of our state-of-the-art DICOM Data Redaction for your healthcare organization. Our feature-rich product offers targeted redaction based on specific DICOM metadata, customizable rules, intelligent contextual analysis, audit trail compliance, redaction preview, and a user-friendly interface.
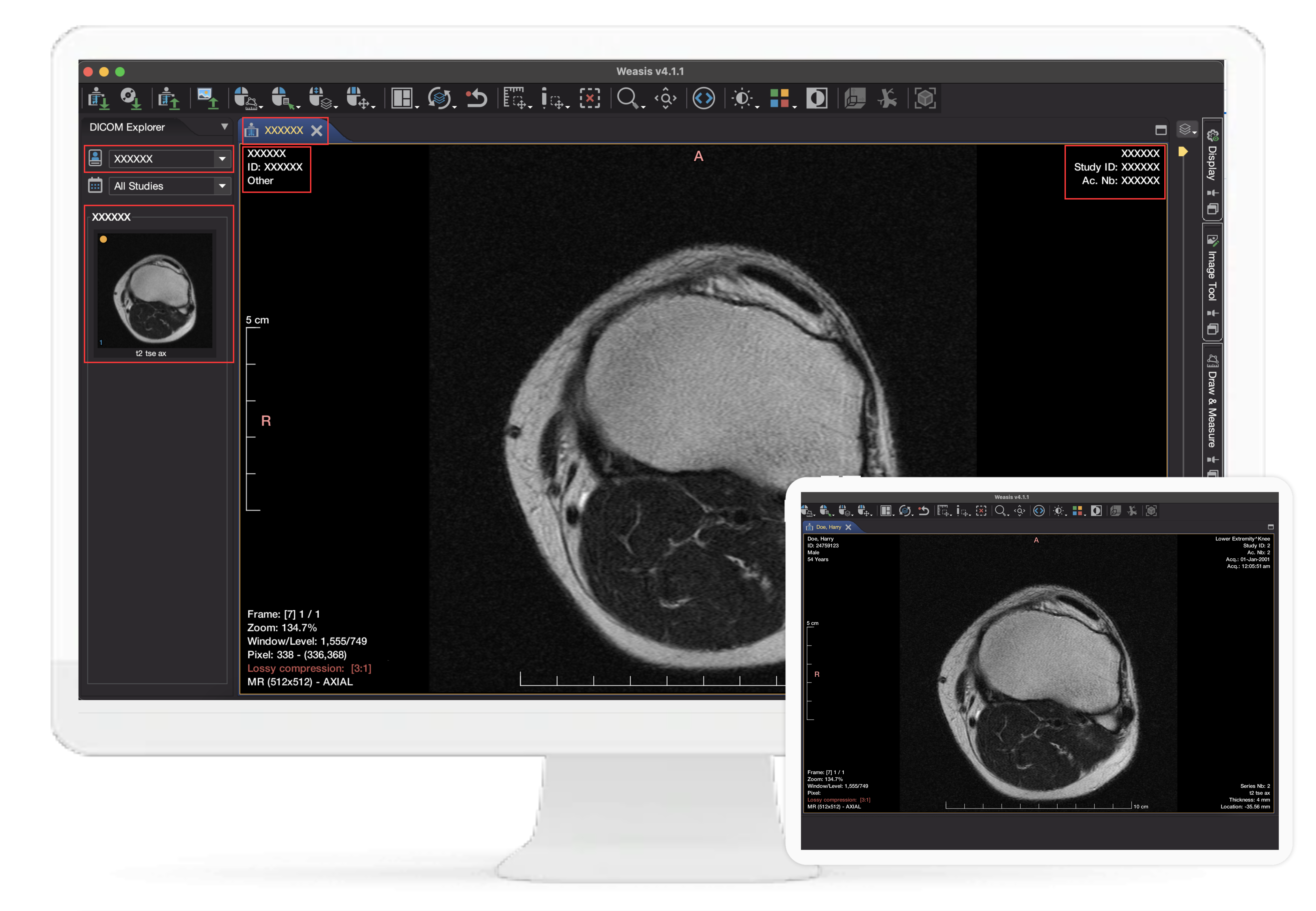
With our solution, you can confidently handle sensitive information while adhering to regulatory requirements. Enhance patient privacy, achieve seamless compliance, and optimize operational efficiency with our advanced DICOM solution.
Key aspects of our solution include

DICOM Data Redaction
clinDICOM.ai enables targeted redaction by leveraging specific DICOM metadata such as patient demographics, study information, imaging modalities, and more. This allows healthcare professionals to precisely identify and redact sensitive information within the DICOM files.

Customizable Redaction Rules
Users have the flexibility to define their own 3R rule - redact, replace or remove based on different DICOM attributes. They can specify keywords or patterns to be redacted, removed, or replaced consistently across a dataset. This customization empowers organizations to align the redaction process with their specific requirements and workflows.

Intelligent Contextual Analysis
Our solution employs advanced contextual analysis techniques to intelligently identify and redact sensitive information within DICOM files. Through machine learning algorithms, it can accurately recognize and protect sensitive data, ensuring patient privacy and data security.

Audit Trail and Compliance
To ensure accountability and compliance with regulatory requirements, clinDICOM.ai maintains a detailed audit trail. This trail tracks all actions performed during the redaction process, providing a comprehensive record of changes made to DICOM files. This feature supports adherence to data privacy regulations and facilitates auditing processes.

Redaction Preview
Our product includes a redaction preview functionality that allows users to review and verify the redacted content before finalizing the changes. This ensures accuracy and gives users the confidence to validate the redaction process, reducing the risk of inadvertent data loss or errors.
Benefits

Enhanced Data Privacy
Safeguard sensitive patient information by accurately redacting specific DICOM metadata, ensuring data privacy and confidentiality.

Time and Cost Efficiency
Automate the redaction process, saving time and effort compared to manual methods, resulting in improved operational efficiency and cost savings.

Accurate Redaction
Intelligent contextual analysis and advanced algorithms ensure precise identification and redaction of sensitive information, minimizing the risk of data exposure.

Redaction Preview
Verify redacted content before finalizing changes, ensuring accuracy and reducing errors in the redaction process.

Streamlined Integration
Our product is designed to integrate seamlessly with popular cloud storage systems like AWS s3, GCP, Azure, any application or storage system. providing users with greater flexibility and control over their clinical trial data.