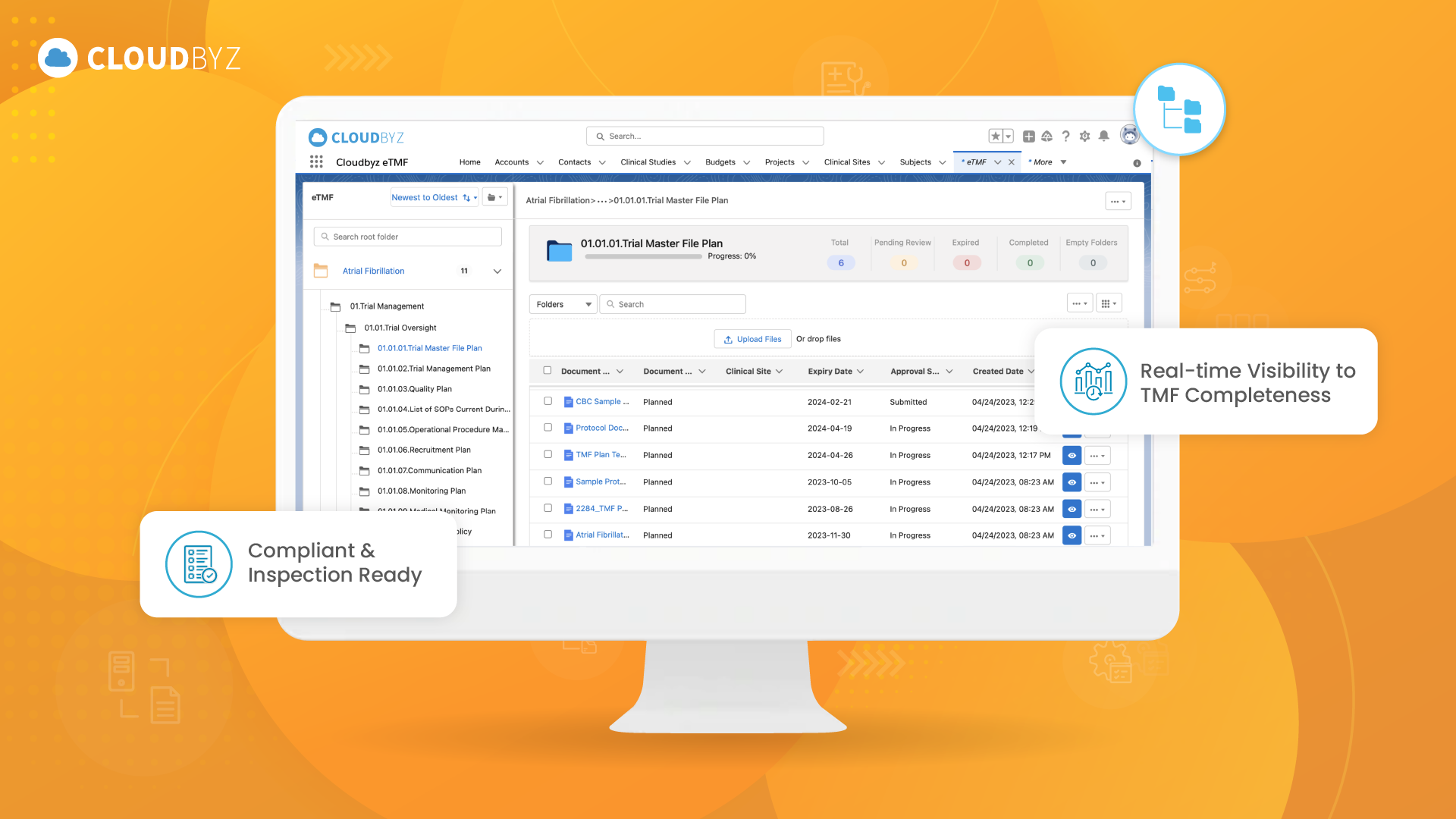
Comprehensive eTMF Capabilities and Evaluation Criteria
The extensive list of eTMF capabilities and evaluation criteria presented in this article, organizations can ensure they choose a system that aligns with their unique requirements and provides a robust, scalable, and secure solution for managing their clinical trial documentation. Such a system will not only streamline processes and enhance compliance but also support long-term success in the ever-evolving world of clinical research.