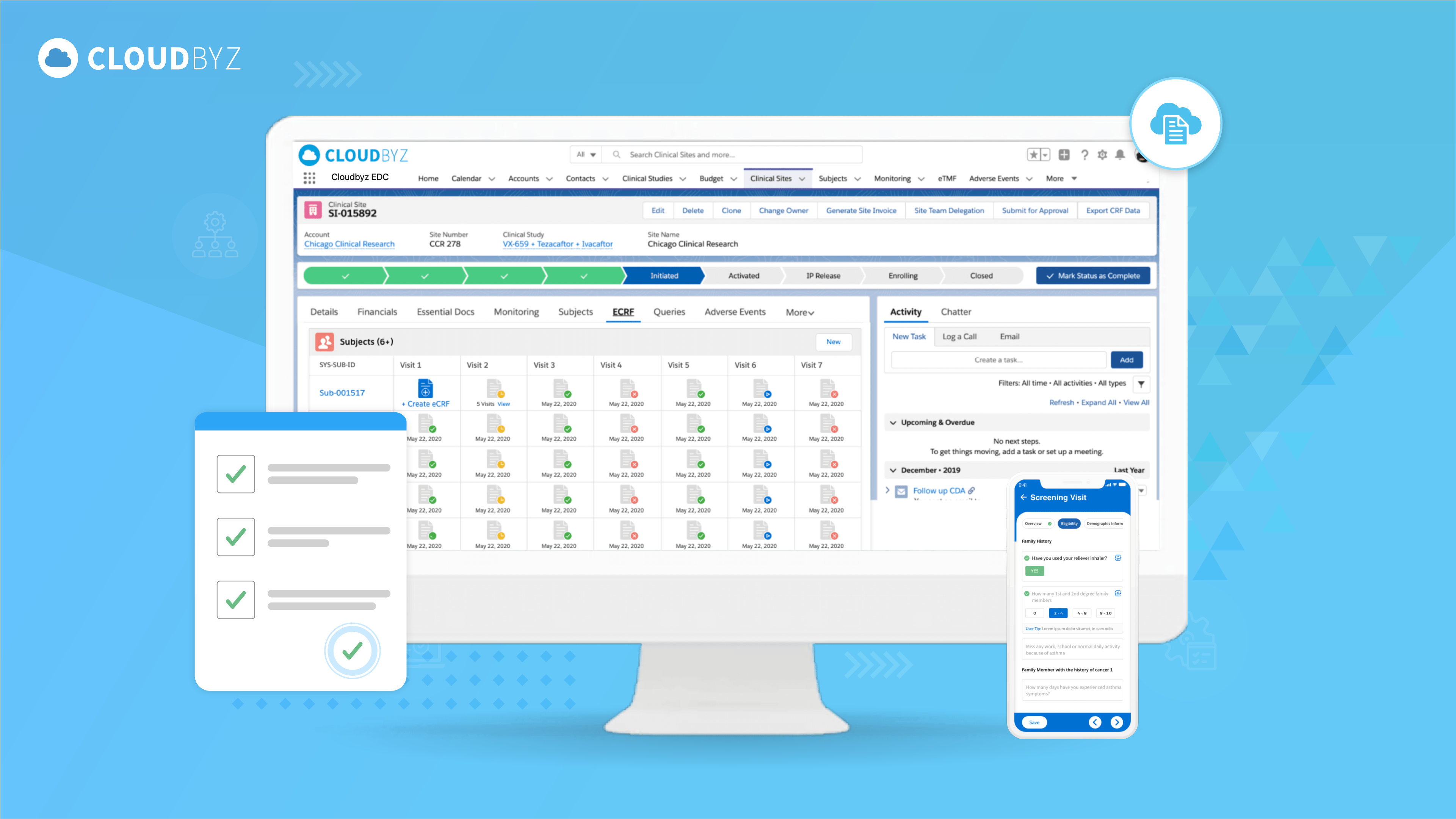
Clinical Trial Data Management Audit Checklist and Best Practices: Ensuring Data Integrity and Compliance
Clinical trial data management audits are essential for maintaining data integrity, regulatory compliance, and the overall success of clinical research. By adhering to a comprehensive audit checklist and implementing best practices, organizations can ensure the highest standards of data quality and ethical conduct. Regular audits not only mitigate risks but also foster continuous improvement and support the advancement of medical science. By prioritizing data management audits, we contribute to the development of safe and effective treatments that benefit patients worldwide.