Comprehensive Guide to Clinical Data Management: Top 50 FAQs Answered for Researchers and Data Managers
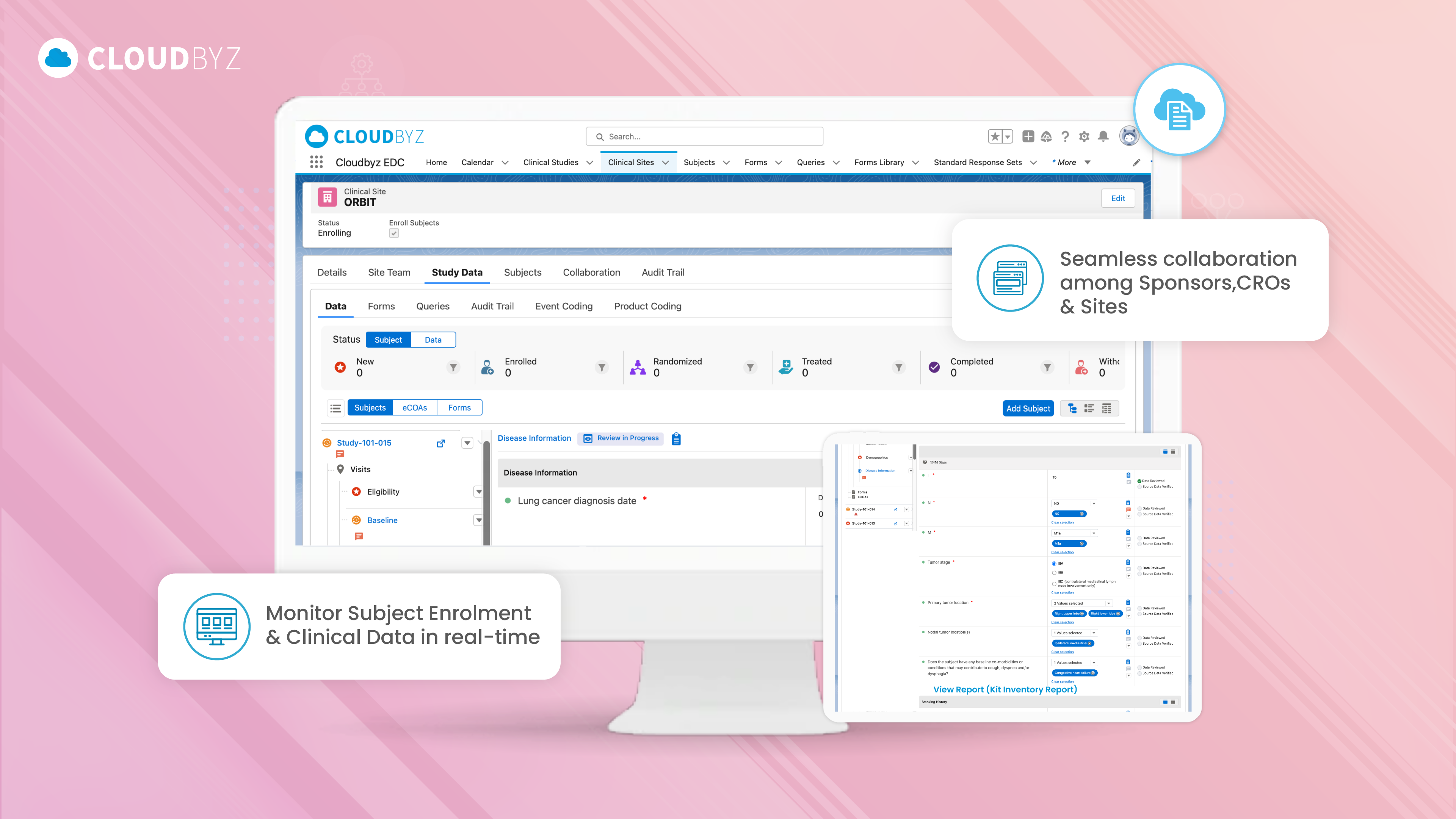
Clinical Data Management (CDM) is a critical component of the clinical research process, ensuring that data collected during trials is accurate, reliable, and compliant with regulatory standards. This comprehensive FAQ has explored the wide-ranging aspects of CDM, from the roles and responsibilities within a CDM team to the technological advancements that are shaping the future of clinical trials.