Comprehensive Guide to Understanding and Implementing Electronic Trial Master Files (eTMFs): FAQ
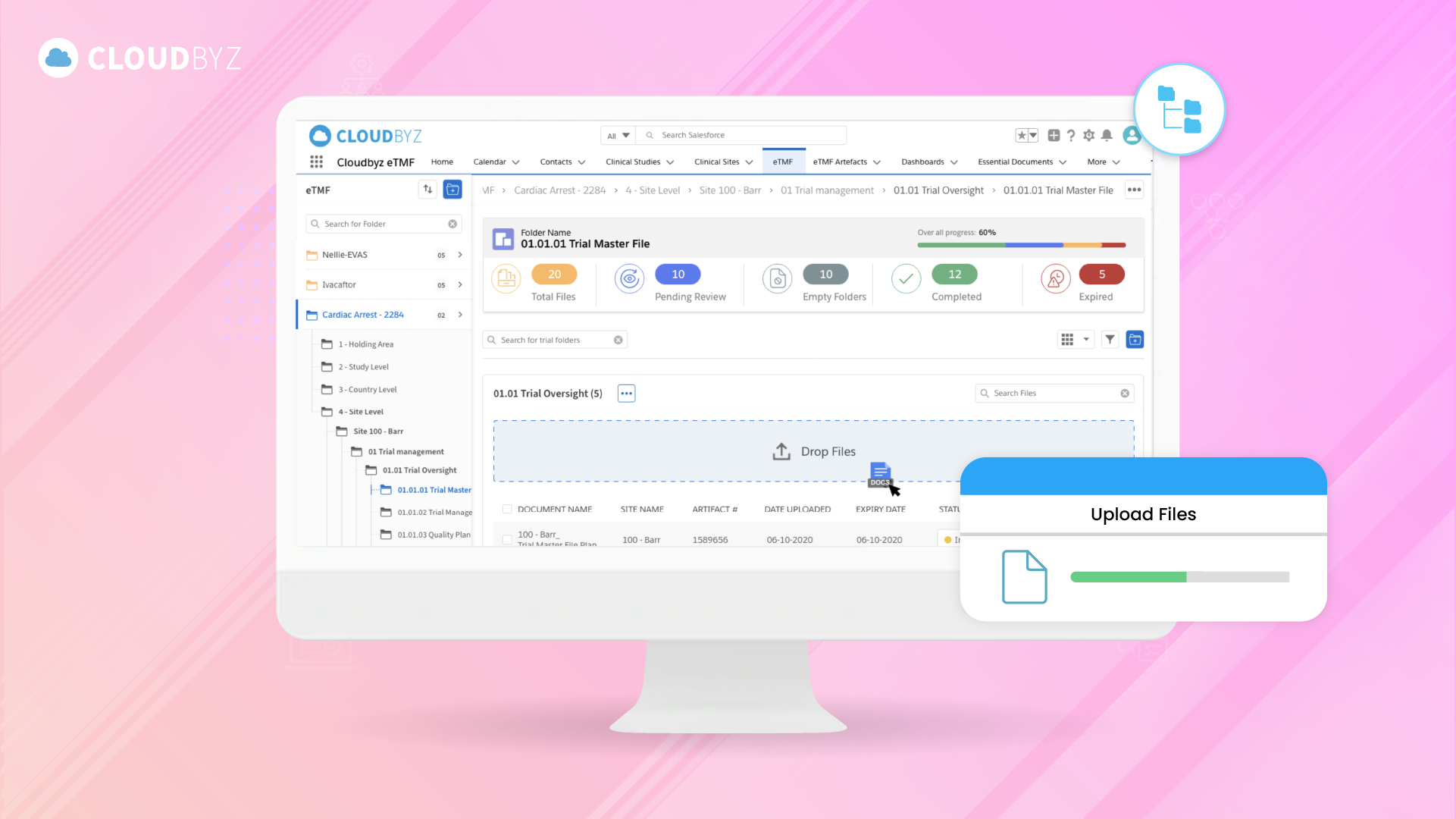
The Electronic Trial Master File (eTMF) represents a significant advancement in the management of clinical trial documentation, offering a streamlined, secure, and compliant solution that meets the growing demands of modern clinical research. By transitioning from traditional paper-based systems to digital eTMF platforms, organizations can enhance their efficiency, improve collaboration across global teams, and ensure that all regulatory requirements are met with precision.