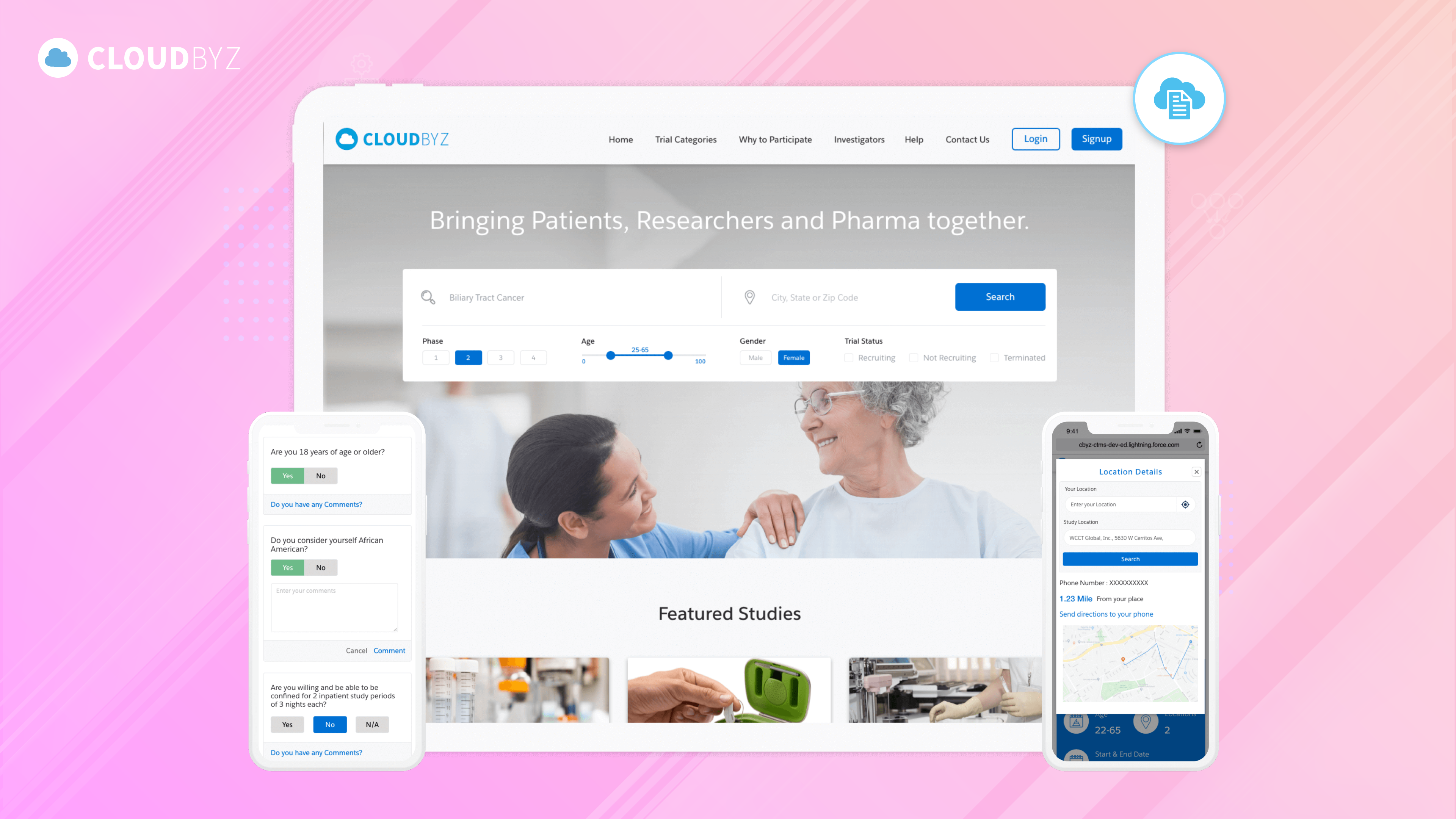
Regulatory Compliance and EDC Systems: Understanding the Requirements
Regulatory compliance is a fundamental aspect of using EDC systems in clinical trials. Adhering to regulations such as FDA 21 CFR Part 11, ICH GCP guidelines, GDPR, and other global standards ensures that clinical trial data is accurate, secure, and trustworthy. By understanding and implementing these requirements, biotech companies can enhance the integrity of their research, protect participant data, and facilitate the approval process for new therapies. As the regulatory landscape continues to evolve, staying informed and proactive in compliance efforts is essential for the successful use of EDC systems in clinical trials.