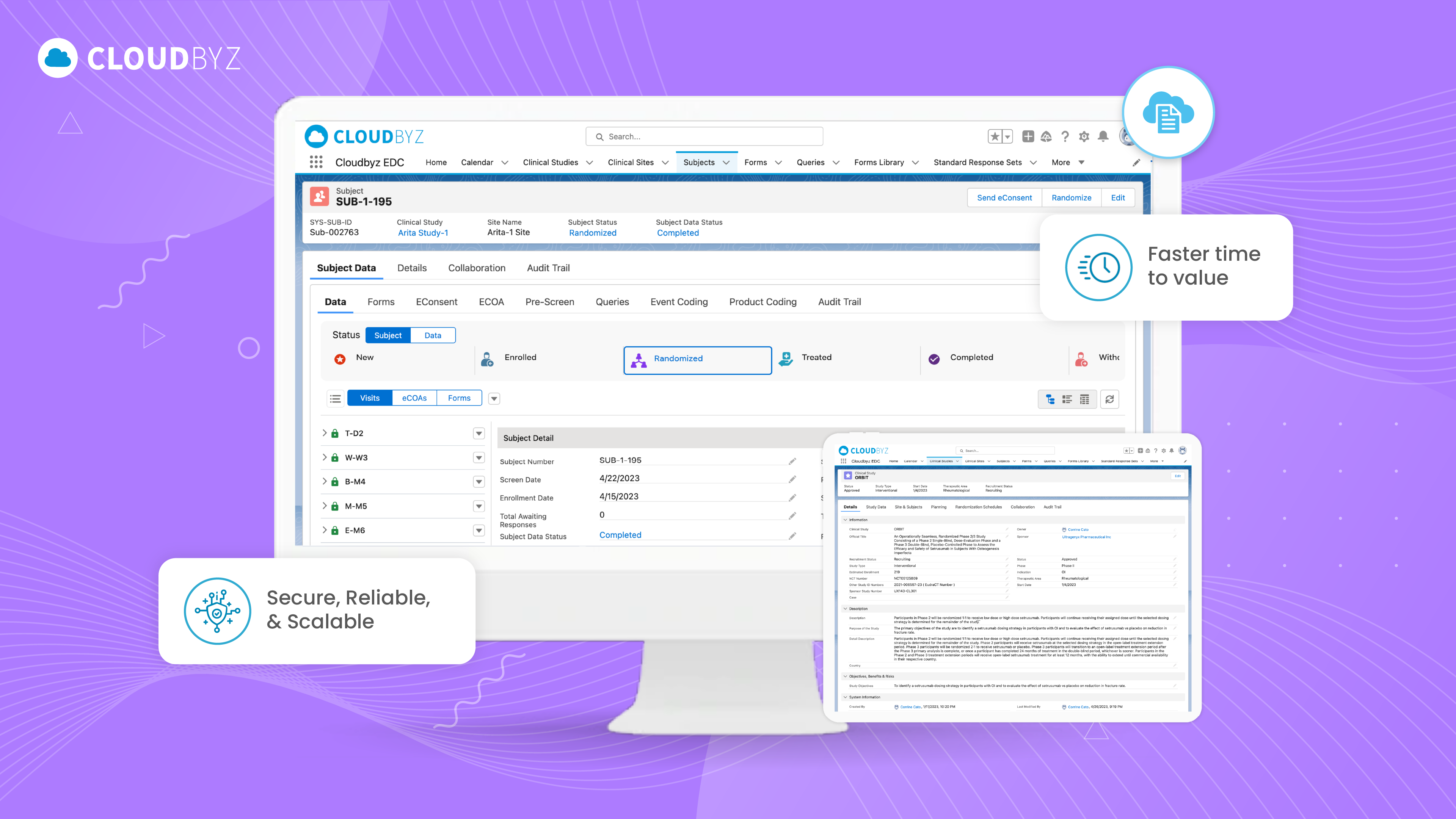
Comprehensive Guide to Electronic Case Report Forms (eCRF): FAQs, Benefits, and Best Practices
Electronic Case Report Forms (eCRFs) have revolutionized the way clinical trials are conducted, offering a robust, efficient, and secure method for capturing and managing trial data. As the clinical research landscape continues to evolve, the adoption of eCRFs has become essential for ensuring data accuracy, compliance with regulatory requirements, and the overall success of clinical studies.