Navigating the Challenges of Post-Market Surveillance with Cloudbyz Safety & Pharmacovigilance
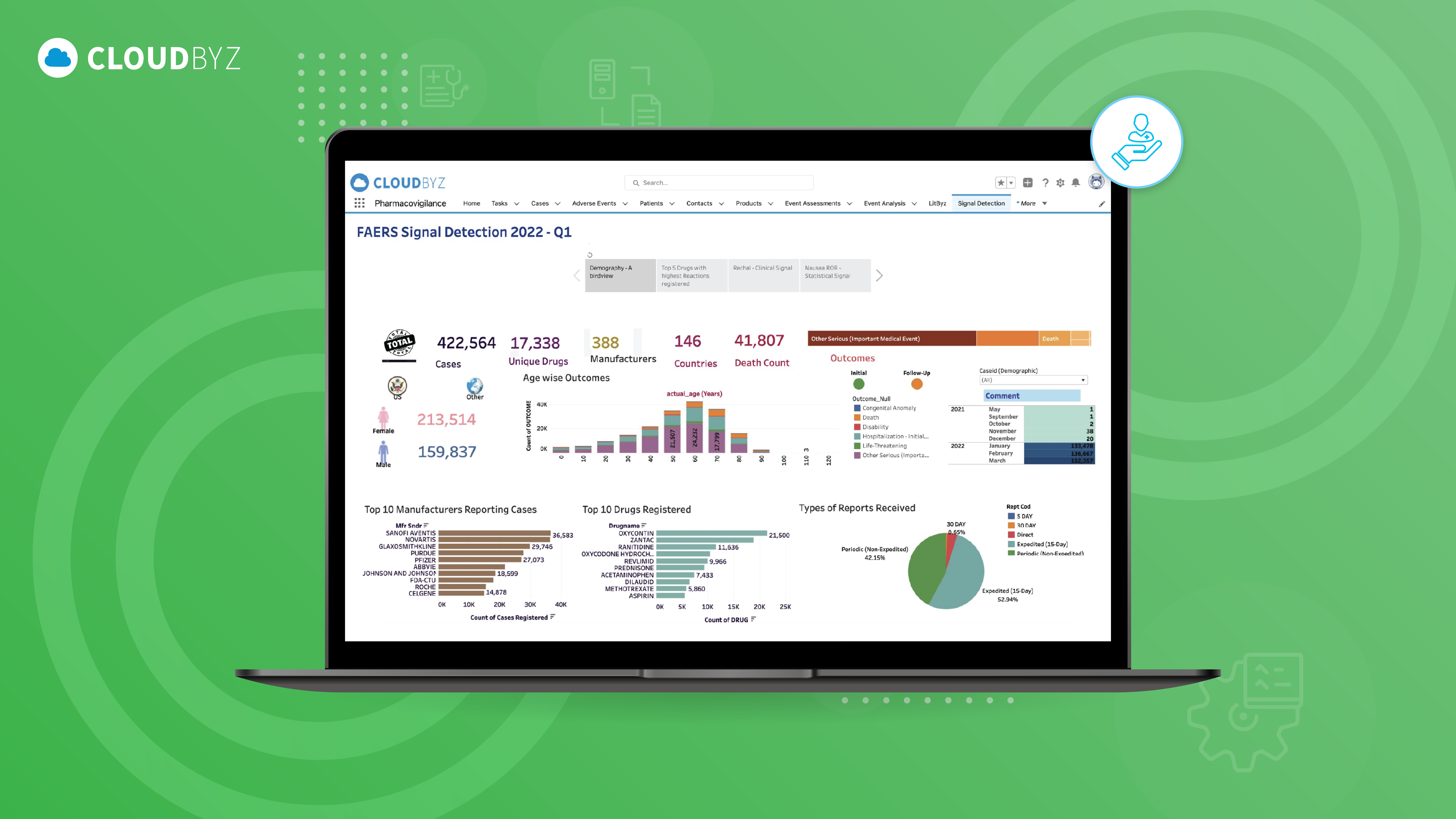
Post-market surveillance is a critical component of pharmacovigilance, essential for ensuring the ongoing safety of pharmaceutical products after they enter the market. The challenges associated with PMS are significant, but with the right tools and strategies, they can be effectively managed.