Ensuring Data Quality: A Comprehensive Guide to Edit Check Specifications during EDC Implementation
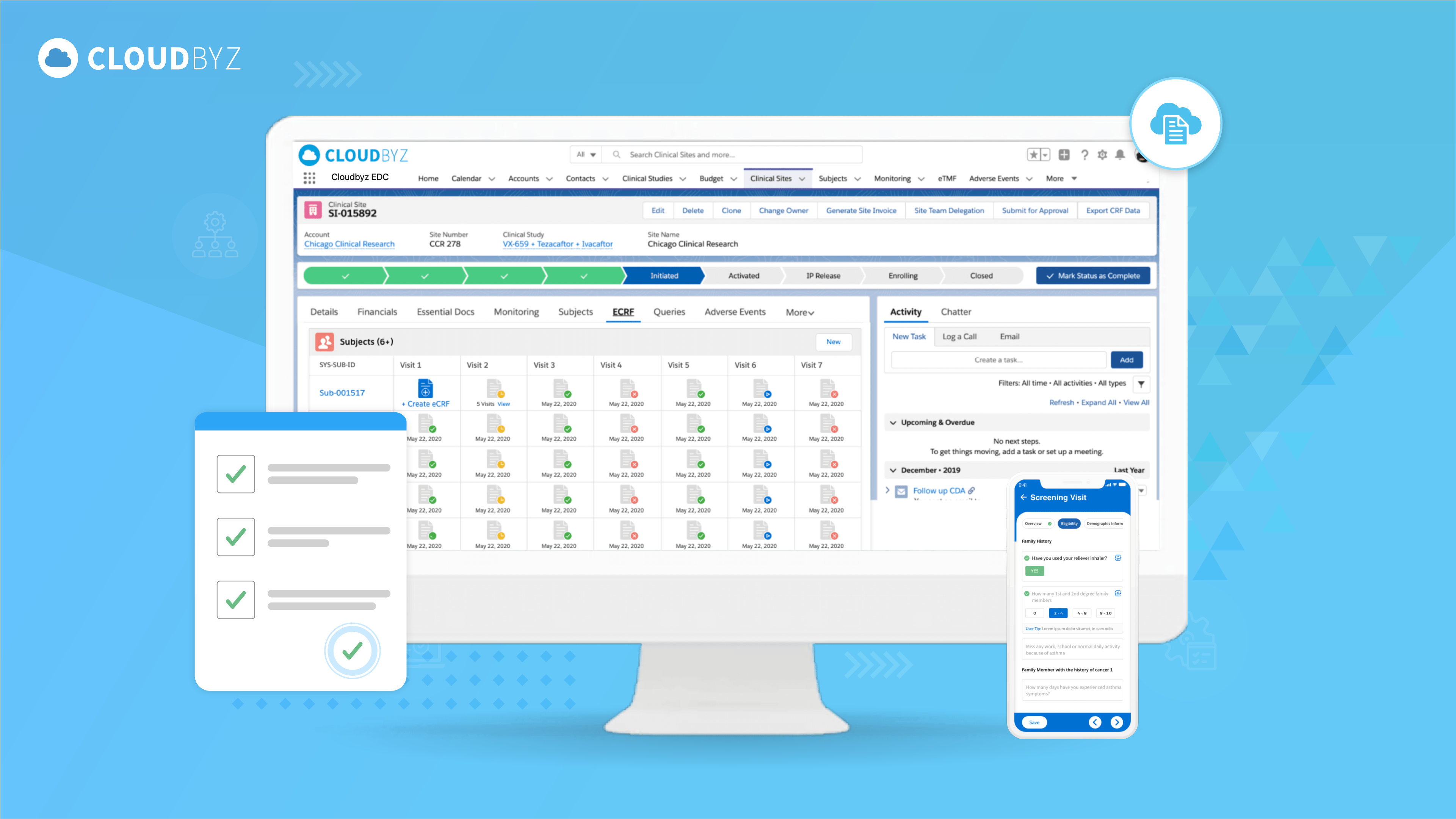
Implementing Edit Check Specifications in EDC systems is a critical aspect of maintaining data quality and integrity in clinical trials. By understanding their importance, developing effective specifications, and addressing the challenges involved, researchers can optimize their EDC implementation and ensure the accuracy and reliability of their clinical trial data.