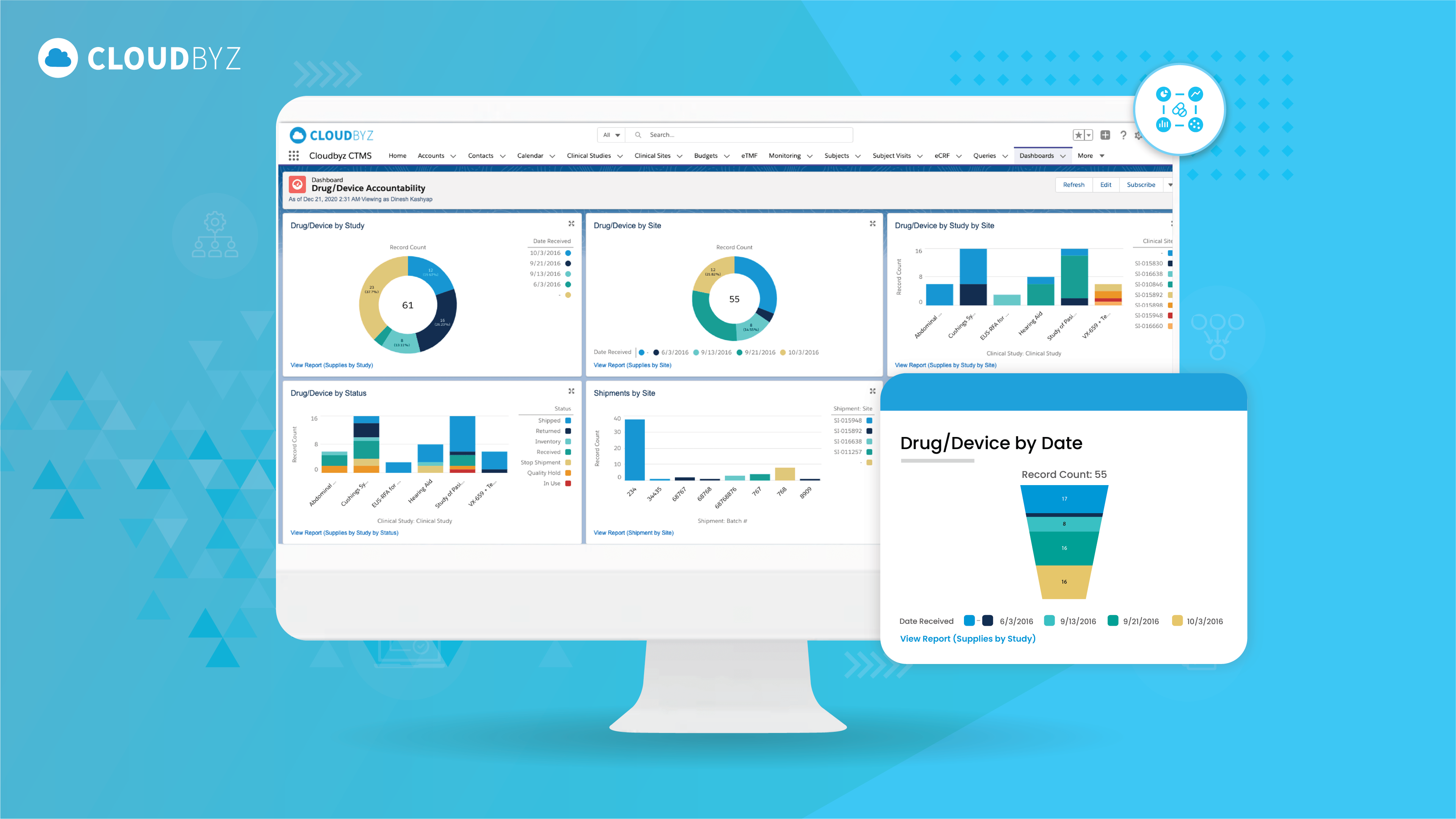
Risk-Based Monitoring (RBM) in Clinical Trial Operations: Best Practices
Risk-Based Monitoring is an essential component of modern clinical trial operations, offering numerous benefits in terms of patient safety, data quality, and resource efficiency. By adopting a systematic approach to identifying, assessing, and mitigating risks, organizations can optimize their monitoring activities and ensure regulatory compliance. The successful implementation of RBM requires the establishment of a risk-based culture, investment in training and education, and collaboration with industry partners and regulatory authorities. By following best practices and adapting to the evolving regulatory landscape, organizations can harness the full potential of RBM to enhance the quality and efficiency of their clinical trial operations.