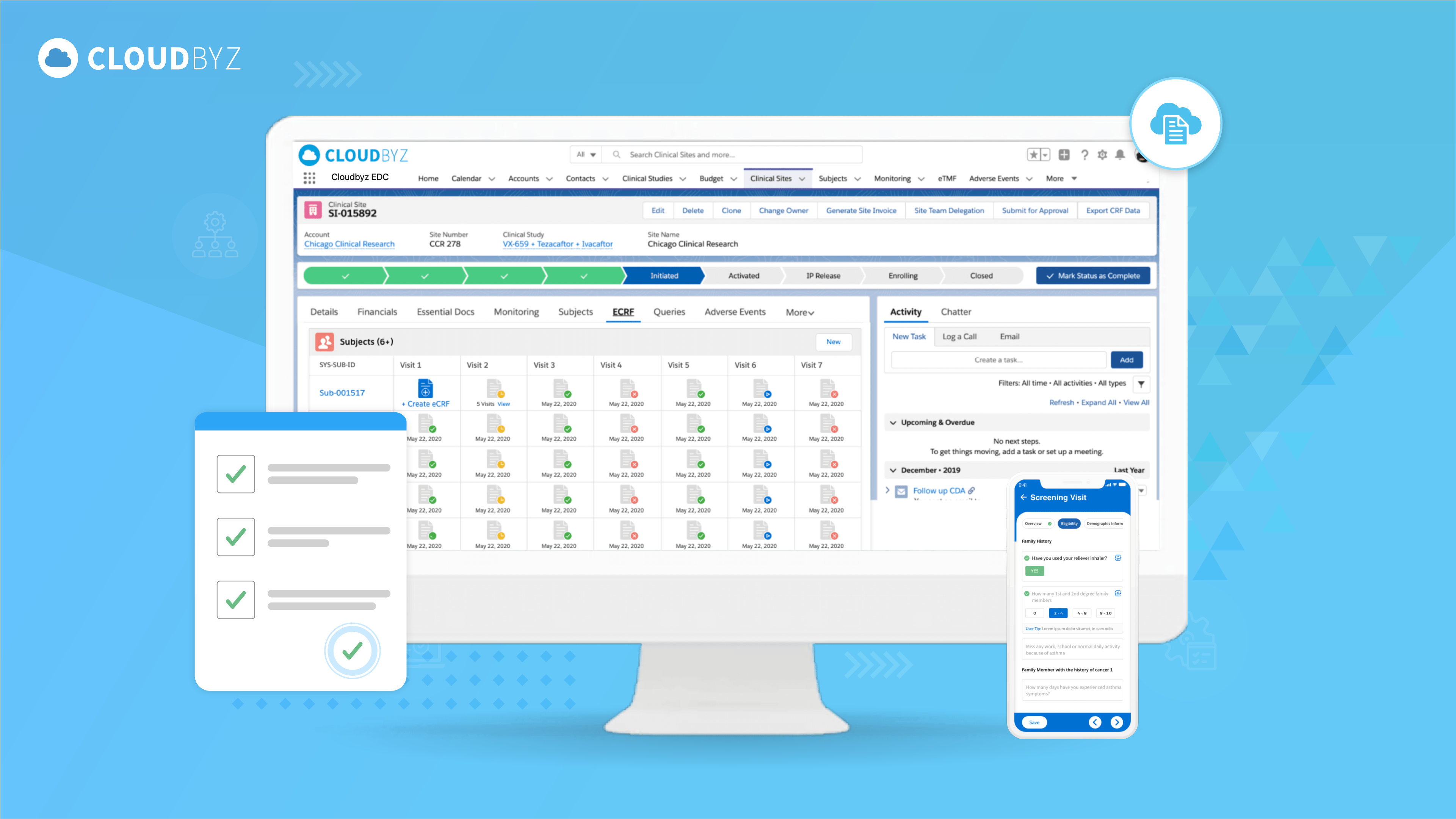
Mastering 21 CFR Part 11 Compliance: A Comprehensive Guide for Clinical Trials in the Digital Age
21 CFR Part 11 stands as a pivotal regulation in the world of clinical trials, establishing the criteria for acceptance of electronic records and electronic signatures by the FDA. Its implementation ensures the reliability, integrity, and security of data throughout the various stages of a clinical trial.