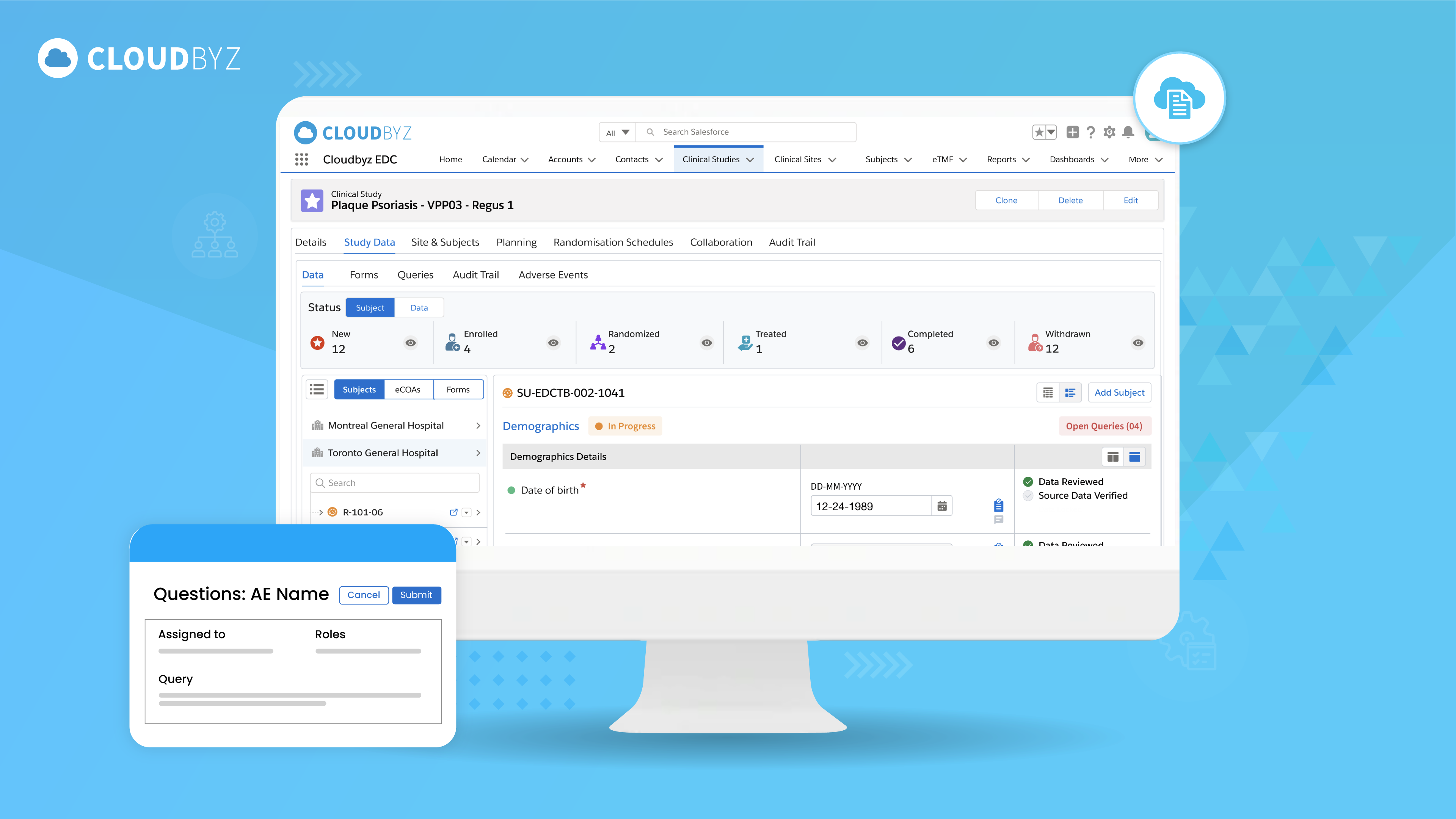
A Comprehensive Guide to Clinical Data Export in SDTM Format from EDC
Exporting clinical trial data in SDTM format from an EDC system is a critical step in the data management process. By following the steps outlined above, researchers and data managers can ensure the accurate representation and compliance of their data with industry standards. Efficiently exporting data in SDTM format facilitates streamlined analysis, interoperability, and regulatory submissions, ultimately contributing to the advancement of medical research.