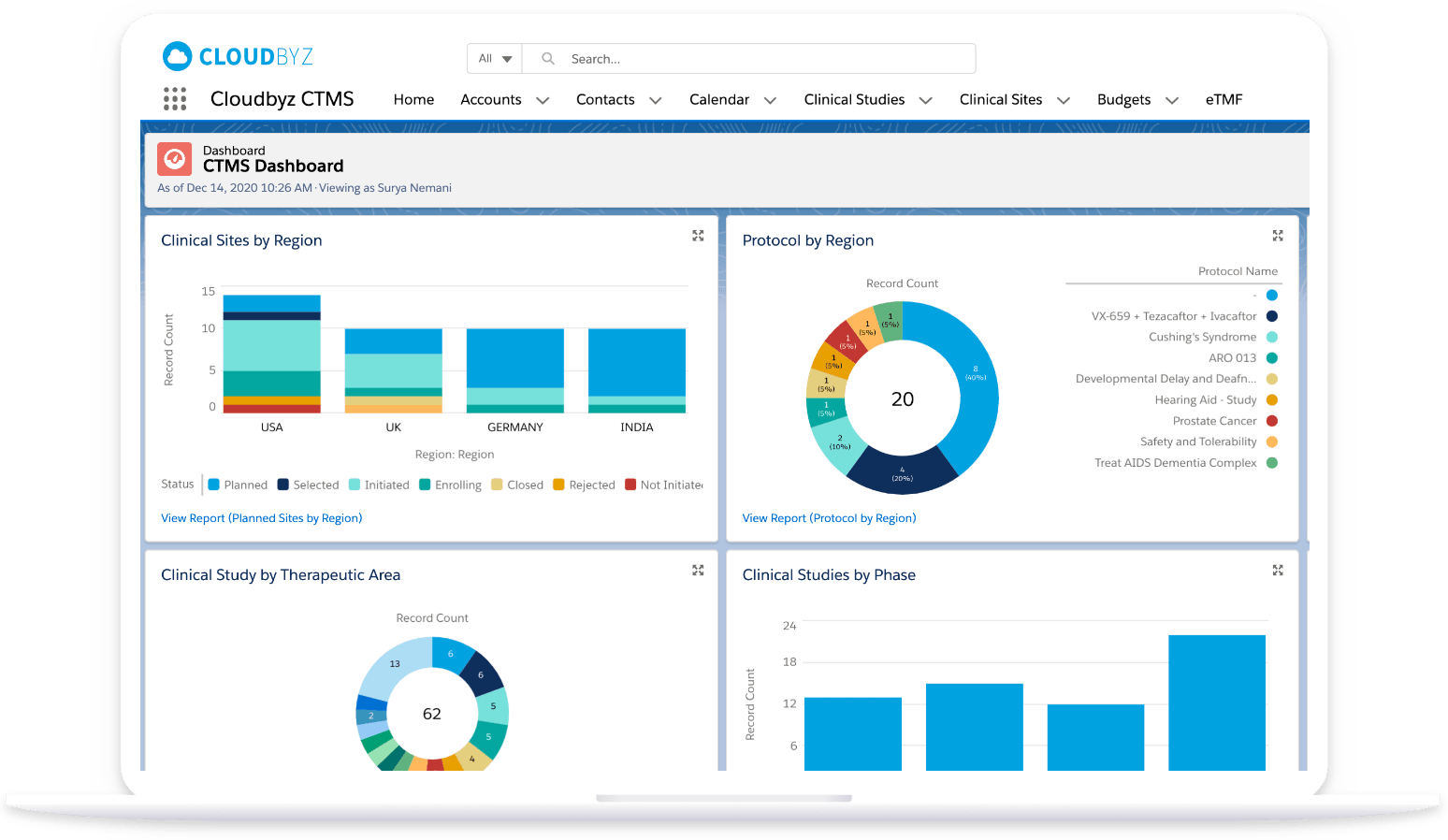
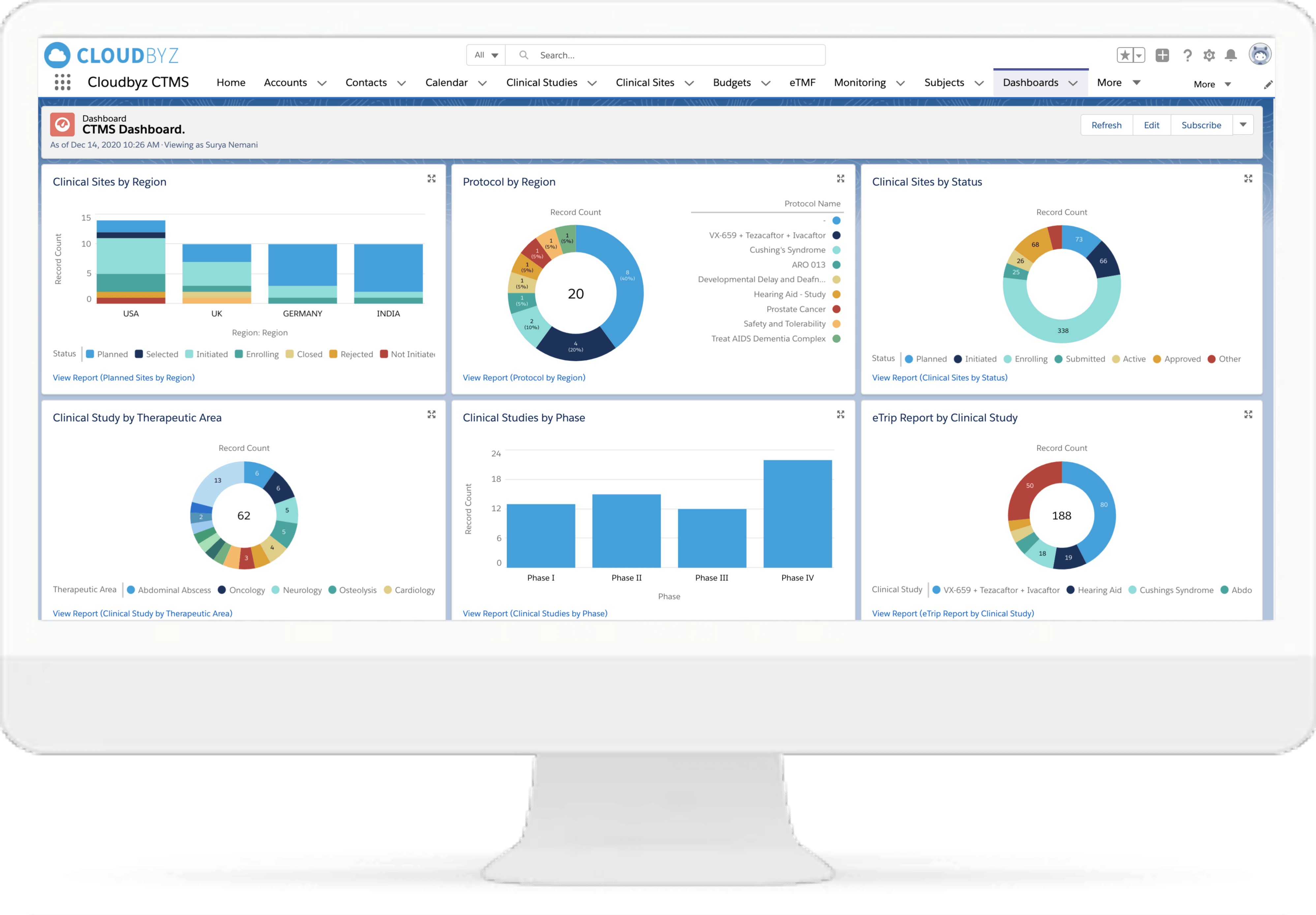
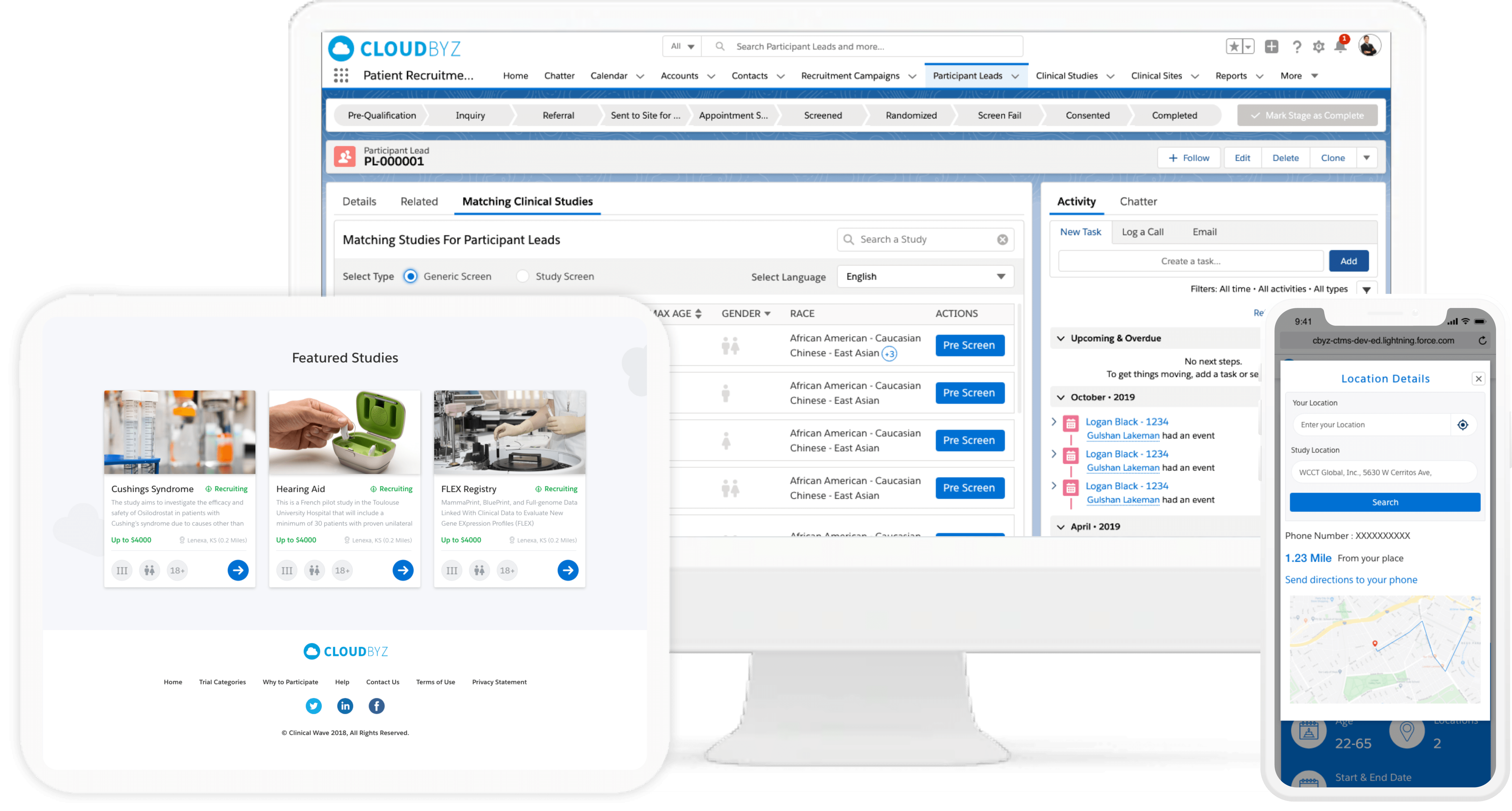
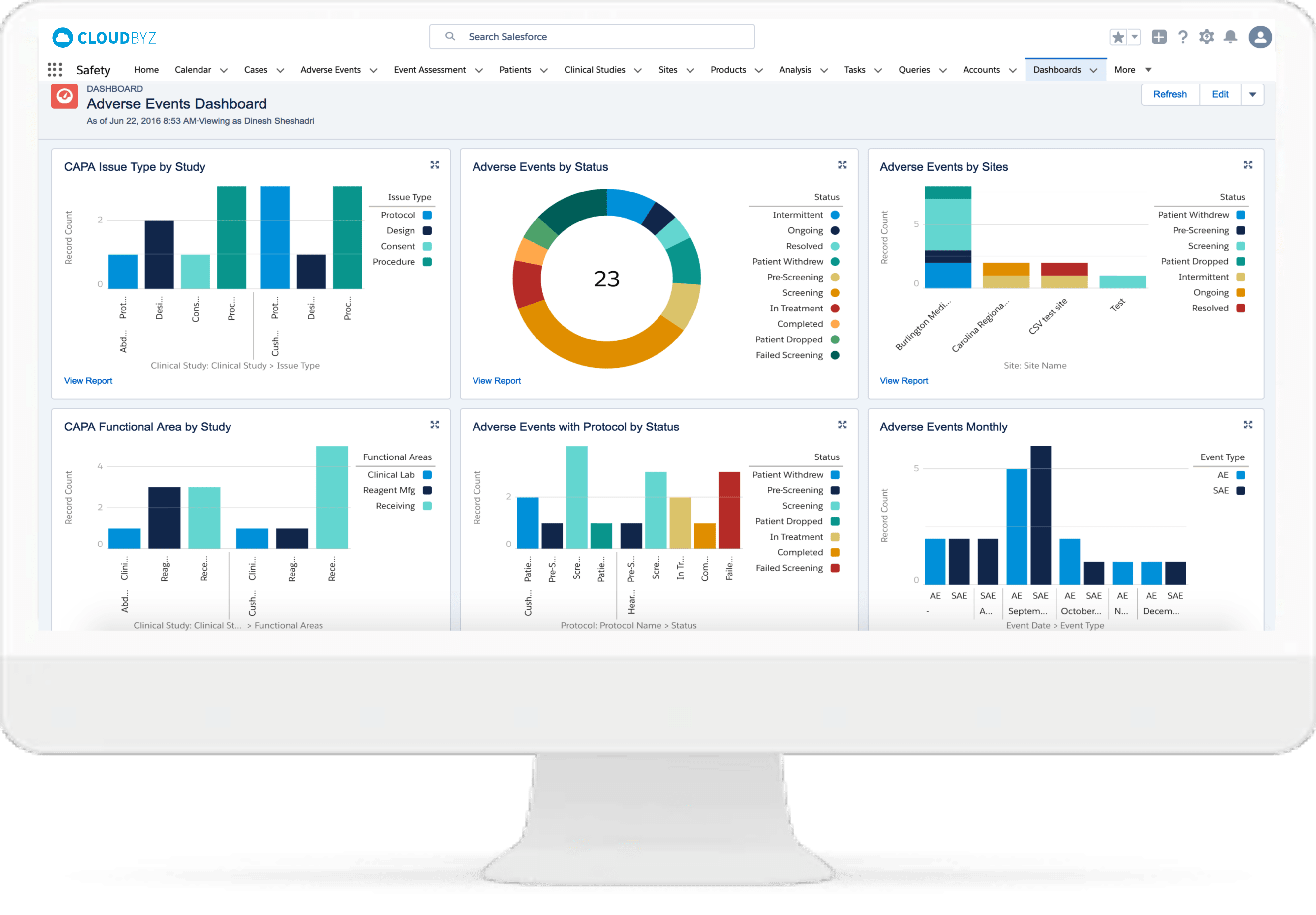
“Have been looking for a solution that does everything in the title for a long time, and with the benefit of the force.com platform, it is even better, particularly with the ability to adapt the tool to our specific needs. We are now easily tracking all applications, the work requests for them, the work done on them, the change management around them. A great tool, and Cloudbyz is very attentive to needs/requests as well. Keep up the good work!”