
Pharmacovigilance Made Easy with Salesforce-Based Platform
Discover how a pharmacovigilance solution built on the Salesforce platform can streamline adverse event reporting, enhance patient safety, and improve compliance.

Hariharan has over 20 years of experience spanning software product development, management consulting and practice/CoE building. He brings proven expertise in helping clients transform their IT landscape in alignment with business strategy. Prior to Cloudbyz, Hari served as VP at CIGNEX Datamatics heading digital platforms and strategic consulting practices. You can find Hariharan on LinkedIn.

Discover how a pharmacovigilance solution built on the Salesforce platform can streamline adverse event reporting, enhance patient safety, and improve compliance.

pharmacovigilance medical coding automation is a promising and rapidly evolving field that has the potential to revolutionize the way pharmacovigilance professionals monitor and assess medication safety. By leveraging the power of AI and machine learning, pharmacovigilance organizations can streamline the medical coding process, improve the accuracy and consistency of ADR reporting, and gain new insights into medication safety trends and risks.

The journey towards ensuring safety in the cosmetics and personal care industry is an intricate one. The stakes are high, given the direct impact of these products on consumers’ health and wellbeing. Therefore, the implementation of robust safety vigilance solutions, such as the Cloudbyz Safety solution built on the Salesforce platform, is not a mere choice—it’s a necessity.
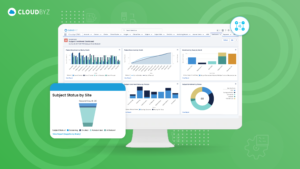
The competitive landscape of the consumer products industry, combined with the growing demand for transparency and scientifically validated claims, has made efficient and effective clinical research a strategic imperative for companies in this space. A unified clinical trial management solution offers a comprehensive answer to this need, streamlining operations, enhancing data management and analytics, improving compliance, and enabling efficient collaboration.
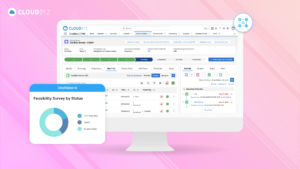
The nutraceutical industry is standing on the precipice of immense growth and transformation. The global inclination towards preventive healthcare and lifestyle management has fueled the demand for clinically-proven, health-enhancing nutraceuticals. As the industry evolves, the necessity of rigorous, streamlined clinical research has never been more apparent.
Unified clinical trial management solutions represent a breakthrough in the face of this necessity, revolutionizing the way nutraceutical companies conduct clinical trials. By integrating various aspects of trials into a single, comprehensive platform, these solutions address numerous challenges, enhance efficiency, and expedite the product development process. They ensure that the focus remains on delivering safe, efficacious products that truly improve consumers’ health and wellbeing.
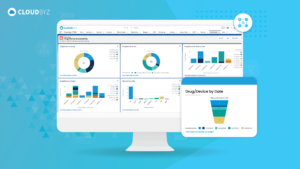
Risk-Based Monitoring is an essential component of modern clinical trial operations, offering numerous benefits in terms of patient safety, data quality, and resource efficiency. By adopting a systematic approach to identifying, assessing, and mitigating risks, organizations can optimize their monitoring activities and ensure regulatory compliance. The successful implementation of RBM requires the establishment of a risk-based culture, investment in training and education, and collaboration with industry partners and regulatory authorities. By following best practices and adapting to the evolving regulatory landscape, organizations can harness the full potential of RBM to enhance the quality and efficiency of their clinical trial operations.

The landscape of clinical research is rapidly transforming, with Real

A platform approach can provide a comprehensive solution for digital
10 considerations in digitizing medical device safety operations to

Electronic-to-business (E2B) gateway is an automated system that facilitates

A unified clinical trial management platform can help biotechnology start-ups to accelerate their path to market by improving efficiency, collaboration, visibility, data management, compliance, and analytics. By leveraging such a platform, biotechnology start-ups can reduce the time and cost of clinical trials, improve the quality of trial data, and increase the chances of getting their therapies approved by regulatory agencies.
At Cloudbyz, our mission is to empower our clients to achieve their business goals by delivering innovative, scalable, and intuitive cloud-based solutions that enable them to streamline their operations, maximize efficiency, and drive growth. We strive to be a trusted partner, dedicated to providing exceptional service, exceptional products, and unparalleled support, while fostering a culture of innovation, collaboration, and excellence in everything we do.
ISO 9001:2015 and ISO 27001:2013 Certified