
The medical device industry is one of the fastest-growing industries globally, with innovation driving the development of new and improved devices. However, getting these devices to market requires extensive clinical trials to ensure their safety and efficacy. The clinical trial process can be time-consuming, with various stakeholders involved, including investigators, sponsors, CROs, and regulatory authorities.
What is a Unified Clinical Trial Management Platform?
A unified clinical trial management platform is a cloud-based software solution that integrates all aspects of clinical trial management into a single, centralized platform. This includes everything from study design and patient recruitment to data collection and analysis. By bringing together all the different components of clinical trial management, a unified platform streamlines the entire process, making it faster, more efficient, and more cost-effective.
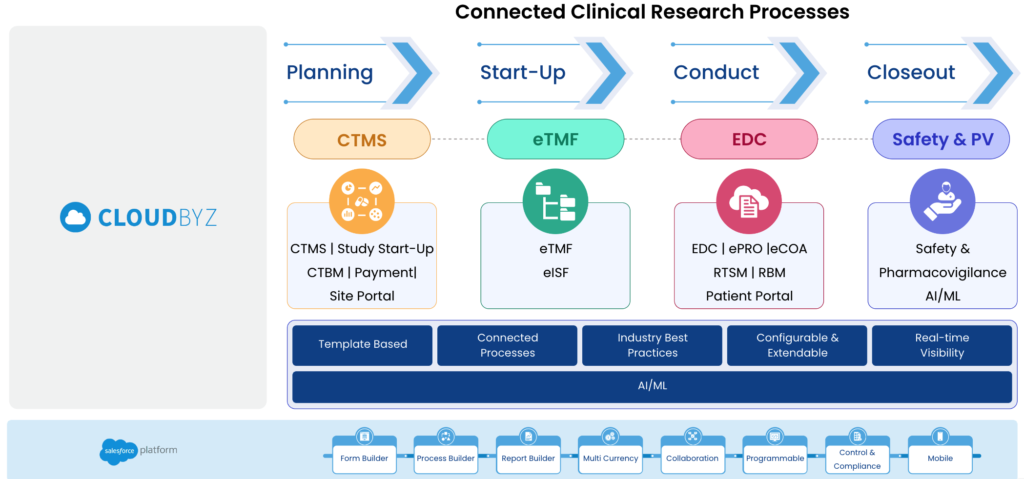
A unified clinical trial platform can help streamline this process and accelerate the launch of medical devices.
Centralized Data Management
A unified clinical trial platform enables the centralization of data management, including patient data, study protocol, clinical data, and regulatory compliance documents. This can significantly reduce the time and effort required to manage and coordinate data across multiple systems, enabling faster decision-making. The platform also provides real-time access to data, enabling stakeholders to make informed decisions quickly.
Real-Time Monitoring
A unified clinical trial platform enables real-time monitoring of clinical trials, including patient enrollment, data collection, and adverse event reporting. This can help identify issues early on and enable faster resolution, resulting in faster study completion. Real-time monitoring also helps sponsors and investigators identify potential patient safety issues, enabling faster response times.
Enhanced Collaboration
A unified clinical trial platform enhances collaboration among stakeholders involved in clinical trial management, including investigators, sponsors, CROs, and regulatory authorities. This can help reduce communication gaps and facilitate faster decision-making. Enhanced collaboration also helps ensure that all stakeholders have access to the same information, reducing the risk of errors and miscommunication.
Automation
A unified clinical trial platform automates many of the manual tasks involved in clinical trial management, including data entry, document management, and regulatory compliance. This can help reduce the time and effort required to manage clinical trials, enabling faster study completion. Automation also helps reduce the risk of errors, improving data accuracy and regulatory compliance.
Improved Compliance
A unified clinical trial platform helps improve compliance with regulatory requirements by providing real-time access to regulatory documents and enabling faster and more accurate reporting of adverse events. The platform also provides a central location for all study documentation, making it easier to manage and ensure compliance.
Faster Study Start-Up
A unified clinical trial platform helps streamline the study start-up process, including site identification, contract negotiation, and study feasibility. This can significantly reduce the time required to initiate clinical trials and enable faster patient enrollment. Faster study start-up also enables sponsors to bring their medical devices to market faster, improving patient access to new and innovative treatments.
In conclusion, a unified clinical trial platform can help accelerate the launch of medical devices by streamlining data management, enhancing collaboration, automating manual tasks, improving compliance, and enabling faster study completion. By providing real-time access to data and enabling faster decision-making, a unified platform helps ensure that medical devices reach the market faster, improving patient access to innovative treatments.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com


