Cloudbyz May CTMS Release Notes
Two new enhancements have been included to both Monitoring Reports and the Feasibility Survey. Also, enhancements in the user interface of eTMF have been made.
Monitoring Reports
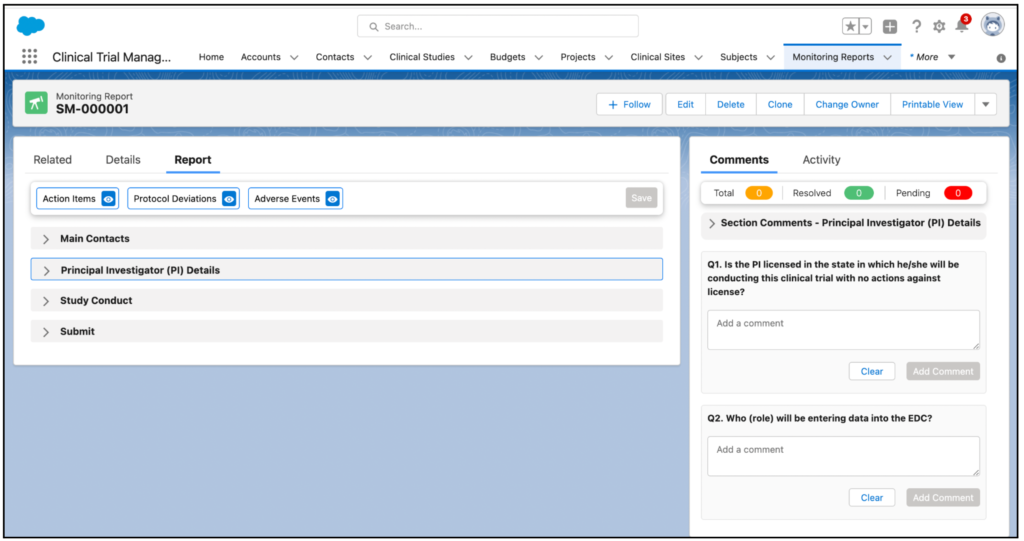
A monitoring report can easily be created by specifying the relevant criteria and simply saving. Other activities monitors can perform include:
- Adding and viewing attendees
- Following up on action items and protocol deviations
- Viewing and linking informed consents, subjects, eCRFs, adverse events, and protocol deviations to the monitoring report
Feasibility Survey
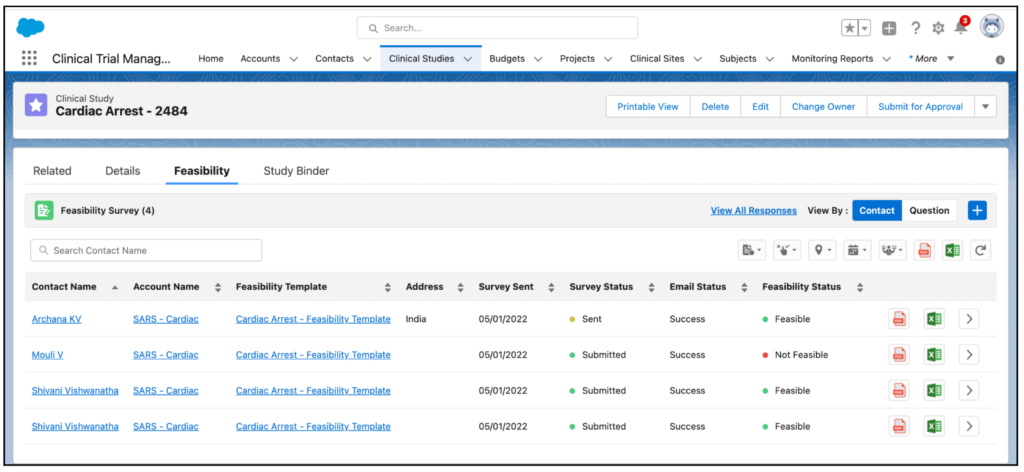
The Feasibility Survey enables you to send feasibility surveys to single or multiple contacts. It also enables you to view and download the survey reports and responses.
The feasibility survey is sent as a link through email. After submitting, the survey link will be open for 24 hours to update responses. If required, you can revisit the survey by clicking the link in the email.
eTMF Enhancements
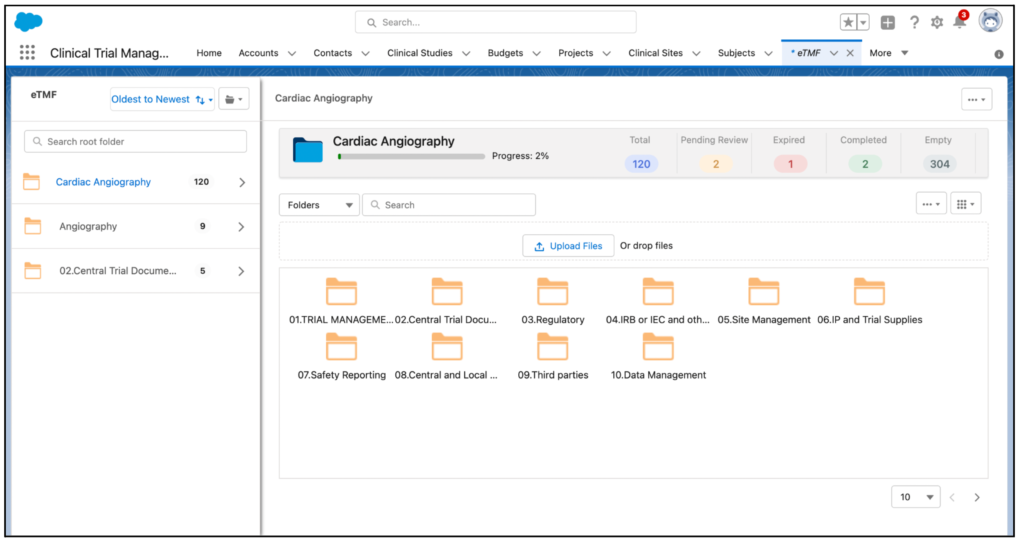
The user interface of eTMF has been enhanced. The dashboard provides a complete view of all the folder data. By just clicking on a folder, it is now possible to view the total number of documents it contains along with the completed, pending, and expired ones on the right pane.
The folder view has been improved with the provision of Thumbnail, List, and Column views. The previous Gear icon, which was used to view the menu, has been replaced with a folder icon.
Now, using the Mass Functionality feature, the users can add, edit, move, and delete single or multiple documents at once. By clicking on a document, the user can preview it and view the metadata, approval history, and audit trail.
For the full release notes see here.


