In the life sciences industry, the management of electronic Trial Master Files (eTMFs) is both a critical and complex task. The eTMF is the backbone of clinical trial documentation, serving as the central repository for all essential documents required to demonstrate compliance with regulatory requirements. As the volume of clinical trial data continues to grow exponentially, the challenges associated with managing, redacting, and extracting critical information from these documents have become increasingly daunting.
Cloudbyz, a leader in innovative clinical trial solutions, offers a comprehensive suite of tools designed to address these challenges head-on. By integrating Cloudbyz eTMF with AI-powered solutions such as ClinRedact and ClinExtract, organizations can enhance their eTMF management processes, streamline operations, and ensure compliance with the ever-evolving regulatory landscape.
The Critical Role of eTMF in Clinical Trials
An eTMF is not just a digital storage solution; it is a dynamic and living document repository that is crucial for the successful conduct of clinical trials. It includes everything from study protocols and informed consent forms to monitoring reports and regulatory approvals. The integrity, accessibility, and accuracy of the eTMF are paramount, as it serves as the primary source of evidence during audits and inspections.
However, managing an eTMF manually is a resource-intensive process that is prone to human error. Traditional methods of handling document redaction and metadata extraction can lead to inconsistencies, compliance risks, and operational inefficiencies. This is where Cloudbyz’s integrated solutions come into play, offering advanced automation capabilities to streamline eTMF management.
Cloudbyz eTMF: A Robust Foundation for Clinical Trial Document Management
Cloudbyz eTMF is a powerful electronic Trial Master File system designed to manage the vast array of documents generated throughout the lifecycle of a clinical trial. It provides a centralized, secure, and compliant repository for all essential documents, ensuring that they are organized, accessible, and audit-ready.
Key features of Cloudbyz eTMF include:
- Centralized Document Repository: Cloudbyz eTMF offers a single source of truth for all trial-related documents, ensuring consistency and accuracy across the board.
- Compliance with DIA TMF Reference Model: The system is aligned with industry standards, ensuring that documents are consistently categorized and managed according to best practices.
- Real-Time Collaboration: Clinical teams can collaborate seamlessly across departments and geographies, with real-time access to the latest documents and updates.
- Audit Readiness: Cloudbyz eTMF maintains comprehensive audit trails and version control, making it easy to demonstrate compliance during regulatory inspections.
While Cloudbyz eTMF provides a strong foundation for managing trial master files, the integration of AI-powered solutions like ClinRedact and ClinExtract takes eTMF management to the next level.
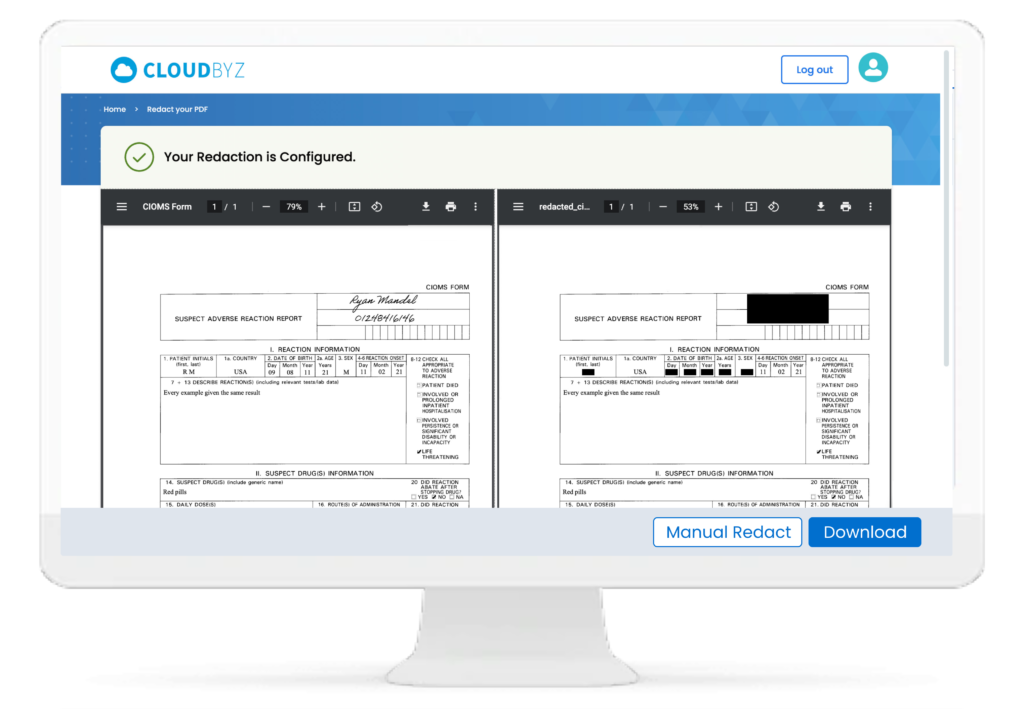
ClinRedact: Automating and Enhancing Document Redaction
Redaction of sensitive information is a critical step in preparing documents for regulatory submission, public disclosure, or sharing with external partners. In the clinical trial context, this often involves the removal of patient identifiers, proprietary information, and other confidential details. Manual redaction processes are time-consuming, error-prone, and resource-intensive.
Cloudbyz ClinRedact automates the document redaction process, leveraging machine learning and natural language processing (NLP) technologies to identify and redact sensitive information with precision. Key benefits of ClinRedact include:
- Accuracy and Consistency: ClinRedact ensures that all sensitive information is accurately redacted across documents, reducing the risk of accidental disclosures.
- Time and Resource Efficiency: Automation significantly reduces the time and resources required for document redaction, allowing clinical teams to focus on higher-value activities.
- Compliance with Global Regulations: ClinRedact supports compliance with international data protection regulations such as GDPR and HIPAA, ensuring that redacted documents meet the highest standards.
- Seamless Integration with Cloudbyz eTMF: Redactions can be performed directly within the eTMF system, streamlining workflows and eliminating the need for manual intervention.
By integrating ClinRedact with Cloudbyz eTMF, organizations can enhance their document redaction processes, ensuring that all trial documents are handled with the utmost care and precision.
ClinExtract: Automating Metadata Extraction for Enhanced Document Management
Metadata is the backbone of effective document management within an eTMF. It includes critical information such as document type, version history, author information, and regulatory status. Accurate metadata ensures that documents can be easily retrieved, tracked, and audited, but manual metadata extraction is both laborious and susceptible to errors.
Cloudbyz ClinExtract automates the process of metadata extraction, utilizing advanced AI and machine learning algorithms to accurately identify and extract metadata from a wide range of document formats. The benefits of ClinExtract include:
- Automated Metadata Extraction: ClinExtract automatically captures and applies metadata, reducing the manual effort involved and ensuring accuracy.
- Improved Document Organization: With accurate metadata applied, documents are organized efficiently within the eTMF, facilitating easy search and retrieval.
- Streamlined Compliance: Accurate metadata is essential for maintaining compliance with regulatory requirements. ClinExtract ensures that all relevant metadata is captured and managed consistently.
- Scalability: ClinExtract is designed to handle large volumes of documents, making it ideal for large-scale clinical trials involving multiple sites and regions.
- Continuous Learning: ClinExtract’s machine learning capabilities allow it to improve over time, becoming more accurate and efficient with each use.
When combined with Cloudbyz eTMF, ClinExtract enhances the overall document management process by ensuring that all metadata is accurately captured and applied, facilitating better organization, retrieval, and compliance.
The Synergy of Cloudbyz ClinRedact, ClinExtract, and eTMF: A Holistic Approach to eTMF Management
The true power of Cloudbyz’s solutions lies in their integration. By combining the capabilities of Cloudbyz eTMF with ClinRedact and ClinExtract, organizations can achieve a holistic approach to eTMF management that addresses the full spectrum of challenges associated with clinical trial documentation.
- Enhanced Efficiency: Automation of both redaction and metadata extraction processes leads to significant time savings and resource efficiency. Clinical teams can redirect their focus from manual document handling to more strategic tasks, such as data analysis and trial oversight.
- Reduced Risk: Automated processes reduce the risk of human error, ensuring that documents are accurately redacted and metadata is consistently applied. This reduction in risk translates to greater compliance with regulatory requirements and a lower likelihood of costly delays.
- Improved Collaboration: With a centralized and well-organized eTMF, teams can collaborate more effectively across departments and geographies. Real-time access to the latest documents and metadata ensures that all stakeholders are working with the most current information.
- Scalability: Cloudbyz’s solutions are designed to scale with the needs of your organization, whether you are managing a single trial or a global portfolio of studies. This scalability ensures that your eTMF management processes remain efficient and effective as your trial operations grow.
- Future-Proofing: As the life sciences industry continues to evolve, Cloudbyz’s solutions are built to adapt to new regulatory requirements, emerging technologies, and growing data volumes. This future-proof approach ensures that your organization remains at the forefront of clinical trial innovation.
Conclusion: Leading the Way in eTMF Management
In an industry where the integrity and accuracy of clinical trial documentation are paramount, Cloudbyz offers a comprehensive and innovative approach to eTMF management. By integrating Cloudbyz eTMF with AI-powered solutions like ClinRedact and ClinExtract, organizations can streamline their document management processes, enhance compliance, and drive greater efficiency across their clinical operations.
The synergy of these tools provides a holistic solution that not only addresses today’s challenges but also positions organizations for future success in a rapidly evolving regulatory landscape. As clinical trials continue to grow in complexity, embracing the power of automation and advanced technology will be essential for maintaining competitive advantage and bringing life-saving therapies to market more quickly and effectively.
With Cloudbyz, life sciences companies can confidently navigate the complexities of eTMF management, ensuring that their clinical trial documentation is always accurate, compliant, and ready for audit.


