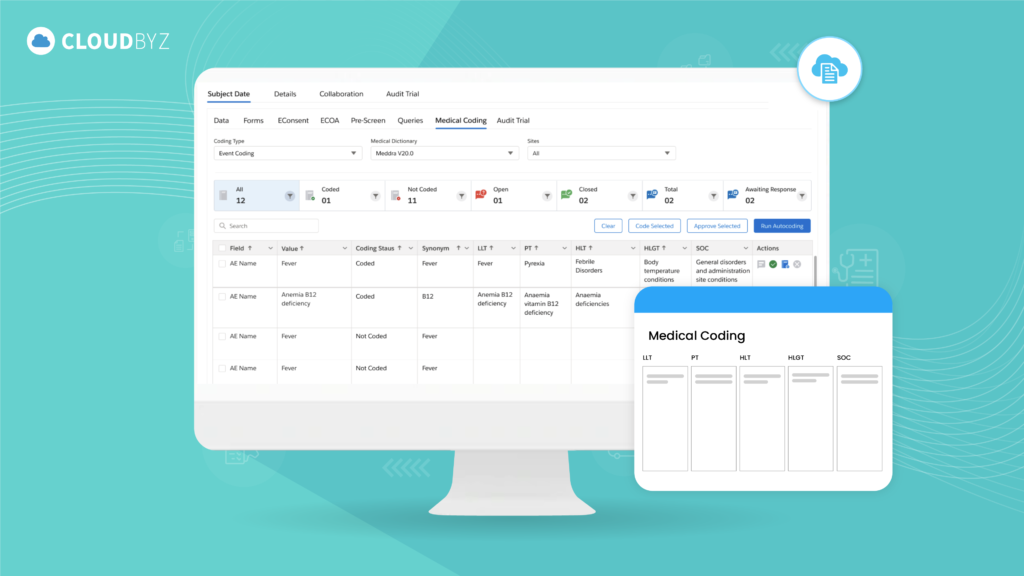
Introduction to Electronic Case Report Forms (eCRF)
In the ever-evolving landscape of clinical trials, the transition from traditional paper-based case report forms to electronic Case Report Forms (eCRFs) has marked a significant advancement in how clinical data is collected, managed, and analyzed. eCRFs have become a cornerstone in modern clinical trials, offering a digital platform that not only streamlines data entry and management but also enhances data accuracy, security, and regulatory compliance.
As clinical trials grow in complexity and scale, the role of eCRFs becomes increasingly critical. These digital forms facilitate real-time data capture, ensure compliance with global regulatory standards, and support the seamless integration of diverse data sources, from lab results to patient-reported outcomes. By automating many of the processes that were once manual and error-prone, eCRFs allow research teams to focus on what truly matters: advancing scientific knowledge and bringing new treatments to market more efficiently.
This FAQ section is designed to provide a comprehensive understanding of eCRFs, addressing common questions and concerns that arise when implementing and using these systems in clinical trials. Whether you are new to eCRFs or looking to deepen your knowledge, these FAQs cover a wide range of topics—from basic definitions and benefits to advanced features and best practices—helping you navigate the complexities of eCRFs with confidence.
Frequently Asked Questions (FAQs) About Electronic Case Report Forms (eCRF)
1. What is an electronic Case Report Form (eCRF)?
An electronic Case Report Form (eCRF) is a digital version of the traditional paper-based Case Report Form used in clinical trials to collect and manage data from participants. eCRFs are designed to streamline the data collection process, ensuring accuracy, consistency, and compliance with regulatory requirements.
2. How does an eCRF differ from a paper CRF?
The primary difference between an eCRF and a paper CRF is that an eCRF is a digital form used to collect and store clinical trial data electronically, while a paper CRF is a physical document. eCRFs offer several advantages over paper CRFs, including real-time data entry, automatic validation checks, easier data monitoring, and improved data security.
3. What are the benefits of using eCRFs in clinical trials?
eCRFs provide numerous benefits, including:
- Efficiency: Streamlines data entry and reduces the time required for data collection.
- Accuracy: Minimizes errors through automated validation checks.
- Data Security: Ensures secure storage and access to clinical trial data.
- Regulatory Compliance: Facilitates adherence to regulatory standards, such as 21 CFR Part 11.
- Real-time Monitoring: Allows for immediate data review and monitoring, enabling faster decision-making.
4. What types of data are typically collected using an eCRF?
An eCRF is used to collect a wide range of data in clinical trials, including:
- Demographic Information: Participant age, gender, and ethnicity.
- Medical History: Past and current medical conditions, treatments, and medications.
- Lab Results: Blood tests, imaging results, and other diagnostic information.
- Adverse Events: Any adverse reactions or side effects experienced by participants.
- Study Outcomes: Primary and secondary endpoints, such as efficacy and safety measures.
5. How is data entered into an eCRF?
Data is typically entered into an eCRF by clinical research staff, such as study coordinators or investigators, directly through a secure web-based interface. Data can also be imported from other electronic systems, such as laboratory information systems (LIS), via integration with the eCRF platform. Real-time validation checks ensure data accuracy and completeness at the point of entry.
6. How does an eCRF ensure data accuracy and consistency?
eCRFs ensure data accuracy and consistency through several features, including:
- Validation Rules: Automated checks that flag discrepancies or out-of-range values.
- Edit Checks: Alerts that prompt users to correct or confirm data entries.
- Audit Trails: Records of all data entries and modifications, including who made the changes and when.
- Standardized Data Fields: Consistent formats for data entry to reduce variability.
7. What are the regulatory requirements for eCRFs?
eCRFs must comply with various regulatory standards, including:
- 21 CFR Part 11: U.S. FDA regulations that establish the criteria for electronic records and electronic signatures.
- Good Clinical Practice (GCP): International guidelines that ensure the ethical and scientific quality of clinical trials.
- Data Protection Laws: Compliance with laws such as GDPR for the protection of personal data.
8. How do eCRFs support remote data collection in decentralized clinical trials?
eCRFs facilitate remote data collection in decentralized trials by allowing participants to enter data from home or other remote locations. eCRFs can be integrated with wearable devices, mobile apps, and other digital tools to capture data directly from participants, ensuring that data collection is continuous and accurate, even outside the clinical setting.
9. How do eCRFs handle adverse event reporting?
Adverse event (AE) reporting is a critical component of eCRFs. The eCRF system allows for the real-time entry and tracking of adverse events, including severity, causality, and outcomes. Automated alerts can be set up to notify study monitors and investigators of any serious or unexpected AEs, ensuring timely intervention and reporting to regulatory authorities.
10. Can eCRFs be customized for different clinical trials?
Yes, eCRFs can be highly customized to meet the specific needs of each clinical trial. Customization options include the design of data fields, the incorporation of study-specific validation rules, and the integration of trial-specific workflows. This flexibility ensures that the eCRF aligns with the unique requirements of each study protocol.
11. How do eCRFs integrate with other clinical trial systems?
eCRFs can integrate with other clinical trial systems, such as Electronic Data Capture (EDC) systems, Clinical Trial Management Systems (CTMS), Laboratory Information Systems (LIS), and Randomization and Trial Supply Management (RTSM) systems. This integration enables seamless data flow between systems, reducing manual data entry and the risk of errors.
12. What is the role of audit trails in eCRFs?
Audit trails in eCRFs provide a detailed log of all data entries, modifications, and access activities. This ensures data integrity by tracking who made changes, what changes were made, and when they were made. Audit trails are essential for regulatory compliance and help in the identification and resolution of data discrepancies.
13. How are eCRFs validated?
eCRFs undergo a rigorous validation process to ensure that they function correctly and meet all regulatory requirements. This process includes:
- User Acceptance Testing (UAT): End users test the eCRF to ensure it meets their needs.
- System Validation: Technical testing to confirm that the eCRF system performs as expected.
- Regulatory Validation: Ensuring that the eCRF complies with standards such as 21 CFR Part 11.
14. How does Cloudbyz eCRF stand out in the market?
Cloudbyz eCRF stands out in the market due to its ease of use, rapid deployment, and seamless integration with the broader Cloudbyz eClinical suite. It offers robust features like real-time data capture, advanced reporting, and dashboard capabilities, all while ensuring compliance with global regulatory standards. Cloudbyz eCRF is designed to support faster study builds, minimize reliance on IT resources, and deliver high-quality data for clinical trials.
15. What are common challenges associated with eCRF implementation, and how can they be overcome?
Common challenges with eCRF implementation include data migration from legacy systems, user adoption, and ensuring system integration with existing trial management tools. These challenges can be overcome by:
- Data Migration: Planning and executing a careful data migration strategy with robust quality checks.
- User Training: Providing comprehensive training and support to ensure users are comfortable with the new system.
- Integration: Ensuring the eCRF system is compatible with other tools used in the trial, such as CTMS and EDC systems, and conducting thorough testing to ensure seamless integration.
16. How does an eCRF handle protocol amendments during a clinical trial?
When a clinical trial protocol is amended, the eCRF must be updated to reflect the changes. This process involves:
- Version Control: Implementing new versions of the eCRF with a clear audit trail of changes.
- User Notifications: Informing users of the updates and providing training if necessary.
- Data Consistency: Ensuring that existing data is compatible with the updated eCRF and managing any discrepancies.
17. Can eCRFs support multiple languages and global trials?
Yes, eCRFs can be configured to support multiple languages, making them suitable for global trials. Multi-language support allows for consistent data collection across different regions and ensures that all participants, regardless of language, can understand and complete the forms accurately.
18. How is participant privacy maintained in eCRFs?
Participant privacy is maintained in eCRFs through several mechanisms:
- Data Encryption: All data entered into the eCRF is encrypted to protect it from unauthorized access.
- Access Controls: Only authorized personnel can access and modify data, with permissions set based on roles.
- De-identification: Personal identifiers can be removed or masked to protect participant privacy while allowing for data analysis.
19. What is the role of eCRFs in adaptive clinical trials?
In adaptive clinical trials, where the trial protocol can be modified based on interim results, eCRFs play a crucial role by:
- Real-time Data Capture: Providing immediate access to trial data to inform adaptive decisions.
- Flexible Design: Allowing for modifications to the eCRF to accommodate changes in the trial protocol.
- Monitoring and Analysis: Enabling continuous monitoring and analysis to support adaptive trial designs.
20. How do eCRFs handle data queries and discrepancies?
eCRFs are equipped with tools to manage data queries and discrepancies efficiently:
- Automated Queries: The system can automatically generate queries when data is missing or inconsistent.
- Manual Queries: Study monitors can manually raise queries for specific data points that require clarification.
- Resolution Workflow: A defined workflow for resolving queries ensures that discrepancies are addressed promptly, with a record of all actions taken.
21. What types of validation checks are commonly used in eCRFs?
Validation checks in eCRFs help ensure data accuracy and may include:
- Range Checks: Ensuring that numeric entries fall within predefined acceptable ranges.
- Consistency Checks: Verifying that related data fields are logically consistent (e.g., date of birth vs. age).
- Mandatory Fields: Ensuring that essential fields are completed before submission.
- Real-time Feedback: Providing immediate feedback to the user if the entered data does not meet the validation criteria.
22. How are eCRF templates created and managed?
eCRF templates are typically created by a combination of clinical and technical teams, based on the specific requirements of the clinical trial. These templates can be managed and reused for future trials, ensuring consistency and reducing setup time. Cloudbyz eCRF, for example, offers customizable templates that can be tailored to meet the specific needs of each study.
23. What role do eCRFs play in ensuring data integrity in clinical trials?
eCRFs are critical for maintaining data integrity by:
- Automated Validations: Reducing the risk of data entry errors.
- Audit Trails: Providing a clear record of all data changes and actions taken.
- Consistent Data Formats: Standardizing data collection to ensure comparability across participants and sites.
- Real-time Monitoring: Allowing for continuous oversight and prompt identification of potential data issues.
24. How do eCRFs facilitate regulatory submissions?
eCRFs facilitate regulatory submissions by ensuring that all collected data is accurate, complete, and compliant with regulatory standards. The system can generate reports and datasets formatted according to regulatory requirements, such as CDISC standards, making the submission process smoother and more efficient.
25. What support is available for troubleshooting issues with eCRFs?
Support for eCRFs typically includes:
- Help Desk: Access to technical support for resolving system issues.
- User Manuals: Comprehensive guides on how to use the eCRF system.
- Training Programs: Ongoing training sessions to keep users updated on system features and best practices.
- Vendor Support: Direct assistance from the eCRF vendor for more complex issues, including system upgrades and customizations.
26. How are changes to eCRF data handled post-entry?
Any changes to eCRF data after initial entry must follow a controlled process:
- Edit Logs: All changes are logged with details on who made the change, what was changed, and when.
- Justification: Users may be required to provide a reason for the change, particularly if the data is critical.
- Audit Trail: An audit trail is maintained to ensure transparency and compliance with regulatory requirements.
27. How do eCRFs handle patient-reported outcomes (PROs)?
eCRFs can be integrated with electronic Patient-Reported Outcome (ePRO) systems, allowing patients to directly enter their data. This data is automatically captured in the eCRF, ensuring that patient-reported outcomes are incorporated into the trial data set without the need for manual entry.
28. Can eCRFs be accessed offline, and how is data synchronized?
Some eCRF systems offer offline access capabilities, where data can be entered without an internet connection. Once the device reconnects to the internet, the data is automatically synchronized with the central database, ensuring no data is lost and all entries are up to date.
29. What are the considerations for selecting an eCRF system?
Key considerations when selecting an eCRF system include:
- Compliance: Ensuring the system meets all relevant regulatory standards.
- Ease of Use: A user-friendly interface that requires minimal training.
- Integration Capabilities: Compatibility with other clinical trial systems and tools.
- Customization: The ability to tailor the eCRF to the specific needs of the trial.
- Vendor Support: Availability of robust support services from the vendor.
- Cost: Consideration of both initial setup costs and ongoing operational expenses.
30. How does Cloudbyz eCRF enhance collaboration in multi-site trials?
Cloudbyz eCRF enhances collaboration in multi-site trials by offering a centralized platform where all sites can access and enter data in real-time. This facilitates consistent data collection, enables efficient monitoring, and ensures that all stakeholders have up-to-date information, regardless of location. The system’s cloud-based architecture also allows for easy scaling as more sites are added to the trial.
31. How do eCRFs handle protocol deviations in clinical trials?
eCRFs are equipped to document and manage protocol deviations efficiently:
- Real-time Alerts: Automatically flag deviations when data is entered that falls outside protocol-defined parameters.
- Deviation Tracking: Maintain a log of all deviations, including details such as the nature of the deviation, the reason for it, and any corrective actions taken.
- Reporting: Generate reports that summarize protocol deviations, which can be used for internal review or regulatory submission.
32. Can eCRFs be used in adaptive and complex trial designs?
Yes, eCRFs are highly adaptable and can be used in complex and adaptive trial designs. They offer:
- Dynamic Form Generation: Ability to adjust data collection forms based on interim results or changes in trial design.
- Modular Design: Allows different modules to be added or removed as the trial progresses.
- Real-time Data Analysis: Supports immediate data analysis to inform adaptive decisions, ensuring the trial remains on course.
33. How do eCRFs support data standardization in global trials?
eCRFs support data standardization across global trials by:
- Uniform Data Fields: Ensuring that all trial sites use the same data fields and formats.
- Global Templates: Providing standardized eCRF templates that can be used across different regions and countries.
- Language Support: Offering multi-language support to ensure consistency in data collection across diverse linguistic groups.
- Regulatory Compliance: Adhering to international standards, such as CDISC, for data collection and reporting.
34. What are the cost implications of implementing an eCRF system?
The cost of implementing an eCRF system can vary depending on several factors, including:
- Licensing Fees: Ongoing costs for using the software, which may be subscription-based or one-time fees.
- Customization: Costs associated with customizing the eCRF to meet specific trial needs.
- Training: Expenses related to training users on the new system.
- Maintenance: Costs for ongoing support, maintenance, and system updates.
- Savings: While the initial costs may be significant, eCRFs often result in long-term savings through reduced data entry errors, faster data collection, and improved efficiency.
35. How does the audit trail functionality in eCRFs contribute to trial integrity?
Audit trails in eCRFs are crucial for maintaining trial integrity by:
- Transparency: Providing a complete record of all data entries, modifications, and deletions, including who made the changes and when.
- Compliance: Ensuring that all actions are documented in compliance with regulatory standards, such as 21 CFR Part 11.
- Traceability: Allowing for easy tracing of any data discrepancies back to their source, facilitating quick resolution.
- Review and Audits: Enabling sponsors and regulatory bodies to review the complete history of data handling during inspections or audits.
36. What role do eCRFs play in ensuring timely data entry and trial monitoring?
eCRFs play a significant role in ensuring timely data entry and monitoring by:
- Real-time Data Capture: Enabling immediate data entry and access for all stakeholders, reducing delays in data availability.
- Automated Reminders: Sending alerts and notifications to site staff to remind them of upcoming or overdue data entry tasks.
- Centralized Monitoring: Allowing trial monitors to view and assess data from all sites in real-time, enabling prompt intervention if issues are identified.
- Data Completeness Checks: Automatically checking for missing or incomplete data, prompting users to address gaps before submission.
37. How are eCRFs used in the management of patient visits and follow-ups?
eCRFs facilitate the management of patient visits and follow-ups by:
- Visit Schedules: Allowing for the creation of visit schedules within the system, including automated reminders for upcoming visits.
- Data Entry Forms: Providing specific eCRF forms tailored to each visit, ensuring that all required data is captured during patient encounters.
- Follow-up Tracking: Tracking patient follow-ups and ensuring that all necessary data is collected over the course of the trial.
- Visit Compliance Monitoring: Monitoring adherence to scheduled visits and flagging any deviations for review.
38. How do eCRFs ensure data security and confidentiality?
Data security and confidentiality in eCRFs are ensured through:
- Encryption: All data is encrypted during transmission and storage to prevent unauthorized access.
- Access Controls: Role-based access controls ensure that only authorized personnel can view or edit specific data fields.
- Data Masking: Sensitive patient data can be masked or de-identified to protect participant privacy.
- Secure Authentication: Multi-factor authentication (MFA) and secure login procedures further protect access to the eCRF system.
- Audit Logs: Comprehensive audit logs track all access and modifications, ensuring accountability.
39. What are the training requirements for users of eCRF systems?
Training requirements for eCRF users typically include:
- System Overview: Introduction to the eCRF system, including its features and navigation.
- Data Entry Procedures: Detailed training on how to enter data correctly and efficiently.
- Validation and Query Resolution: Guidance on how to handle validation checks and resolve data queries.
- Compliance Training: Ensuring that users understand regulatory requirements related to eCRF use, such as 21 CFR Part 11 compliance.
- Ongoing Support: Access to user manuals, help desks, and refresher courses to ensure continuous proficiency.
40. How do eCRFs contribute to reducing the overall time of clinical trials?
eCRFs contribute to reducing the overall time of clinical trials by:
- Faster Data Collection: Real-time data entry and automated validations accelerate the data collection process.
- Improved Data Quality: Reduced data entry errors minimize the need for time-consuming data cleaning and re-entry.
- Efficient Monitoring: Centralized monitoring allows for quicker identification and resolution of issues, preventing delays.
- Streamlined Reporting: Automated report generation facilitates faster data analysis and decision-making.
- Reduced Paperwork: Eliminating the need for physical documents and manual data entry shortens timelines for data collection and review.
41. What is the process for resolving discrepancies in eCRF data?
The process for resolving discrepancies in eCRF data typically involves:
- Automated Alerts: The system flags discrepancies, such as out-of-range values or inconsistent data entries.
- Manual Review: Data managers or monitors review the flagged discrepancies and determine the appropriate course of action.
- Query Generation: If necessary, queries are raised and sent to the site for clarification or correction.
- Resolution Documentation: Once resolved, the actions taken to address the discrepancy are documented in the audit trail.
- Final Review: A final review ensures that all discrepancies have been resolved before data lock.
42. Can eCRFs support real-time collaboration between study teams?
Yes, eCRFs are designed to support real-time collaboration between study teams by:
- Centralized Access: Providing a single platform where all team members can access and contribute to the trial data.
- Role-Based Permissions: Allowing different team members to perform specific tasks based on their roles.
- Communication Tools: Integrating communication tools for team discussions and issue resolution within the platform.
- Real-time Updates: Ensuring that all team members have access to the latest data and updates as they happen.
43. How are eCRFs designed to meet the needs of different therapeutic areas?
eCRFs can be tailored to meet the specific needs of different therapeutic areas by:
- Custom Data Fields: Creating data fields that are relevant to the specific therapeutic area, such as oncology or cardiology.
- Specialized Templates: Utilizing templates that are designed for particular types of trials or conditions.
- Regulatory Considerations: Ensuring that the eCRF meets therapeutic area-specific regulatory requirements.
- Outcome Measures: Including specific outcome measures and assessments that are standard for the therapeutic area.
44. How do eCRFs handle large-scale, multi-center trials?
eCRFs are well-suited for managing large-scale, multi-center trials by:
- Scalability: The ability to handle large volumes of data and support numerous trial sites.
- Standardized Forms: Ensuring consistency in data collection across all sites with standardized eCRF forms.
- Centralized Data Management: Allowing data from all sites to be managed and monitored centrally.
- Site-Specific Customization: Accommodating site-specific needs while maintaining overall trial consistency.
- Real-time Site Monitoring: Enabling real-time monitoring of site performance and data quality across all centers.
45. What considerations are important for eCRF data migration?
When migrating data to an eCRF system, important considerations include:
- Data Integrity: Ensuring that all data is accurately transferred without loss or corruption.
- Validation: Running validation checks before and after migration to confirm data accuracy.
- Historical Data: Deciding whether to migrate historical data and how it will be integrated into the new system.
- Compliance: Ensuring that the migration process complies with all regulatory requirements, including maintaining audit trails.
- Training: Providing training to staff on the new system and the implications of the migration process.
46. How do eCRFs handle data export for statistical analysis?
eCRFs facilitate data export for statistical analysis by:
- Standard Formats: Exporting data in standard formats like CSV, SAS, or CDISC SDTM, which are compatible with statistical software.
- Custom Export Options: Allowing customization of the data export to include specific variables, patient populations, or time points.
- Data Integrity Checks: Ensuring that exported data maintains its integrity and matches the original data entered in the eCRF.
- Secure Transfer: Using secure methods for data transfer to prevent unauthorized access during export.
47. Can eCRFs be used for real-time data visualization and reporting?
Yes, eCRFs often include features for real-time data visualization and reporting:
- Dashboards: Providing real-time dashboards that display key metrics, such as patient enrollment, data completeness, and adverse events.
- Custom Reports: Allowing users to generate custom reports based on specific criteria or data points.
- Graphical Representations: Offering graphical representations of data trends, such as line graphs, bar charts, and pie charts, to facilitate data interpretation.
- Automated Reports: Automatically generating and distributing regular reports to stakeholders based on predefined schedules.
48. How do eCRFs ensure compliance with Good Clinical Practice (GCP) guidelines?
eCRFs ensure compliance with GCP guidelines through:
- Data Accuracy: Automated validation checks ensure that data is accurate and complete.
- Audit Trails: Comprehensive audit trails track all changes to the data, maintaining transparency and accountability.
- Role-Based Access: Ensuring that only authorized personnel can enter or modify data, consistent with their role in the trial.
- Documentation: Providing thorough documentation of all eCRF processes, including user training and system validation, to demonstrate adherence to GCP.
49. What are the backup and disaster recovery options for eCRF systems?
Backup and disaster recovery options for eCRF systems typically include:
- Regular Backups: Automated daily or hourly backups to ensure data is not lost.
- Cloud Storage: Secure cloud-based storage solutions that provide redundancy and high availability.
- Disaster Recovery Plans: Comprehensive disaster recovery plans that outline the steps to restore data and system functionality in the event of a failure.
- Testing: Regular testing of backup and recovery processes to ensure they work effectively when needed.
50. How do eCRFs handle patient consent and electronic signatures?
eCRFs manage patient consent and electronic signatures by:
- Integrated eConsent: Incorporating electronic consent forms (eConsent) that participants can review and sign digitally.
- Compliance with 21 CFR Part 11: Ensuring that electronic signatures meet regulatory requirements for validity and authenticity.
- Audit Trails: Keeping a record of all signed consent forms, including timestamps and user details, within the audit trail.
- Document Management: Storing signed consent forms securely within the eCRF system for easy access and retrieval during audits or inspections.
51. Can eCRFs be adapted for post-market surveillance studies?
Yes, eCRFs can be adapted for post-market surveillance (PMS) studies by:
- Custom Data Fields: Including fields specific to post-market data, such as long-term safety and effectiveness measures.
- Adverse Event Reporting: Enabling detailed tracking and reporting of adverse events over extended periods.
- Patient Follow-Up: Facilitating ongoing patient follow-up and data collection long after the initial clinical trial has ended.
- Regulatory Reporting: Streamlining the process of reporting post-market data to regulatory authorities.
52. What are the advantages of using cloud-based eCRFs?
Cloud-based eCRFs offer several advantages, including:
- Scalability: Easily scalable to accommodate large trials with multiple sites or complex data requirements.
- Accessibility: Accessible from any location with internet access, enabling global collaboration.
- Automatic Updates: Regular system updates without the need for manual intervention, ensuring that the software remains up-to-date with the latest features and security patches.
- Cost-Effectiveness: Reduced need for on-site IT infrastructure and support, leading to lower overall costs.
53. How do eCRFs integrate with wearable devices and mobile apps?
eCRFs can integrate with wearable devices and mobile apps by:
- Real-time Data Collection: Automatically capturing data from wearable devices, such as heart rate, activity levels, and other biometrics, and feeding it directly into the eCRF.
- Mobile Data Entry: Allowing participants to enter data through mobile apps, which is then synced with the eCRF.
- Remote Monitoring: Enabling remote monitoring of participants’ health and adherence to the trial protocol through connected devices.
- Data Integration: Combining data from multiple sources (e.g., wearables, mobile apps, and manual entry) into a single, unified dataset within the eCRF.
54. How do eCRFs support blinding in clinical trials?
eCRFs support blinding (masking) in clinical trials by:
- Role-Based Access: Restricting access to certain data (e.g., treatment allocation) so that blinded personnel cannot view it.
- Blinded Data Entry: Allowing data to be entered without revealing which treatment group the participant is in.
- Blinded Reports: Generating reports that exclude information that could unblind the trial, ensuring that analysis and review can be conducted without bias.
- Data Masking: Masking specific fields in the eCRF to prevent unblinded personnel from accessing sensitive information.
55. What are the common validation procedures for eCRF systems?
Common validation procedures for eCRF systems include:
- System Validation: Ensuring that the eCRF system meets all functional requirements and performs as expected.
- User Acceptance Testing (UAT): Involving end-users in testing to confirm that the system meets their needs and expectations.
- Performance Testing: Assessing the system’s performance under various conditions, including high data loads and concurrent user access.
- Regulatory Compliance Testing: Verifying that the eCRF system complies with relevant regulatory requirements, such as 21 CFR Part 11.
- Documentation: Creating comprehensive documentation of the validation process, including test plans, test cases, and results.
56. How do eCRFs support data monitoring committees (DMCs) in clinical trials?
eCRFs support Data Monitoring Committees (DMCs) by:
- Real-time Data Access: Providing DMC members with real-time access to trial data, enabling them to monitor progress and safety.
- Blinded and Unblinded Data Views: Offering both blinded and unblinded data views, depending on the DMC’s role and the trial design.
- Interim Analysis: Facilitating interim analyses by allowing DMC members to review data at predefined points in the trial.
- Automated Alerts: Sending automated alerts to DMC members if safety thresholds are crossed or if other significant events occur.
57. Can eCRFs be used in conjunction with Electronic Data Capture (EDC) systems?
Yes, eCRFs are often integrated with Electronic Data Capture (EDC) systems:
- Data Synchronization: Ensuring that data entered in the eCRF is automatically synced with the EDC system, maintaining consistency across platforms.
- Seamless Workflow: Streamlining the workflow between data entry in the eCRF and broader data management in the EDC.
- Unified Reporting: Combining data from both systems to generate comprehensive reports and dashboards.
- Regulatory Compliance: Ensuring that the integration complies with regulatory standards, maintaining data integrity and security.
58. How do eCRFs manage protocol compliance in clinical trials?
eCRFs manage protocol compliance by:
- Validation Checks: Automatically checking data against protocol requirements to ensure compliance.
- Protocol Deviation Alerts: Flagging any deviations from the protocol, such as missed visits or incorrect dosing, and alerting the appropriate personnel.
- Audit Trails: Documenting all protocol-related activities and any deviations in the audit trail for later review.
- Compliance Monitoring: Providing tools for real-time monitoring of protocol adherence across all sites, enabling proactive management.
59. What are the key considerations for eCRF design?
Key considerations for eCRF design include:
- User-Friendliness: Ensuring that the interface is intuitive and easy to navigate for users at all levels of technical expertise.
- Data Integrity: Designing the eCRF with built-in validation checks to maintain data accuracy and consistency.
- Customization: Allowing for customization to meet the specific needs of the trial, including unique data fields and workflows.
- Regulatory Compliance: Incorporating features that ensure compliance with relevant regulatory standards, such as 21 CFR Part 11.
- Scalability: Ensuring that the eCRF can scale to accommodate large and complex trials, including multi-site studies.
60. How do eCRFs manage data cleaning and query resolution?
eCRFs manage data cleaning and query resolution by:
- Automated Data Checks: Automatically identifying inconsistencies, out-of-range values, and missing data for review.
- Query Generation: Allowing study monitors to generate queries that are sent directly to the site staff for resolution.
- Query Tracking: Tracking all queries and their resolution status within the system, ensuring that no issues are overlooked.
- Data Correction: Enabling authorized users to correct data directly in the eCRF while maintaining an audit trail of all changes.
61. How do eCRFs handle longitudinal data collection in clinical trials?
eCRFs are well-suited for longitudinal data collection by:
- Visit Schedules: Managing and organizing data across multiple visits and time points.
- Time-Point Specific Forms: Designing forms specific to each visit or time point to capture relevant data.
- Data Trends Analysis: Facilitating the analysis of trends over time, such as changes in patient symptoms or lab results.
- Cohort Management: Allowing the tracking and management of patient cohorts over the duration of the study, including long-term follow-ups.
62. What are the considerations for integrating eCRFs with laboratory information systems (LIS)?
When integrating eCRFs with Laboratory Information Systems (LIS), key considerations include:
- Data Format Compatibility: Ensuring that data formats are compatible between the eCRF and LIS to facilitate seamless data exchange.
- Real-time Data Import: Enabling real-time or near-real-time import of lab results into the eCRF to keep trial data up-to-date.
- Error Handling: Implementing error-handling procedures to manage any discrepancies or errors in lab data.
- Regulatory Compliance: Ensuring that the integration meets all regulatory requirements, particularly around data integrity and audit trails.
63. How do eCRFs support real-world evidence (RWE) studies?
eCRFs support real-world evidence (RWE) studies by:
- Flexible Data Collection: Allowing for the collection of diverse types of real-world data, including patient-reported outcomes, medical records, and observational data.
- Integration with EHRs: Facilitating integration with electronic health records (EHRs) to capture real-world clinical data.
- Data Analysis Tools: Offering tools for the analysis of real-world data to generate evidence on the effectiveness and safety of interventions in everyday clinical practice.
- Scalability: Supporting large-scale RWE studies across multiple sites and populations.
64. Can eCRFs handle multi-arm clinical trials, and how?
Yes, eCRFs are capable of handling multi-arm clinical trials by:
- Custom Forms for Each Arm: Designing specific eCRF forms for each treatment arm to capture relevant data.
- Randomization Integration: Integrating with randomization systems to ensure that patients are assigned to the correct arm and that data is captured accordingly.
- Comparative Analysis: Enabling the comparison of data across different arms, including efficacy and safety outcomes.
- Blinding Support: Maintaining blinding where required by ensuring that certain data is hidden from specific users.
65. How do eCRFs manage adverse event (AE) tracking and reporting?
eCRFs manage adverse event (AE) tracking and reporting through:
- Standardized AE Forms: Providing standardized forms for AE data entry, ensuring consistent data capture across the trial.
- Severity and Causality Assessment: Allowing for the entry of details on the severity and causality of AEs.
- Real-time Alerts: Sending real-time alerts to study monitors or investigators when a serious adverse event (SAE) is reported.
- Regulatory Reporting: Generating reports that are formatted for submission to regulatory authorities, including detailed AE summaries.
66. What are the options for customizing eCRF workflows?
eCRF workflows can be customized to fit the specific needs of a clinical trial by:
- Custom Approval Processes: Defining custom approval workflows for data entry, review, and sign-off.
- Form Sequencing: Customizing the sequence in which eCRF forms are completed, based on the study protocol.
- Conditional Logic: Implementing conditional logic within forms, where certain fields or sections only appear based on previous responses.
- Automated Notifications: Setting up automated notifications and reminders based on the workflow, such as alerts for missing data or upcoming deadlines.
67. How do eCRFs support quality assurance (QA) in clinical trials?
eCRFs support quality assurance (QA) by:
- Validation Rules: Implementing strict validation rules that ensure data is accurate and complete at the point of entry.
- Audit Trails: Maintaining a detailed audit trail of all data entries and changes, which can be reviewed as part of the QA process.
- Real-time Monitoring: Enabling real-time monitoring of data entry and site performance, allowing QA teams to identify and address issues quickly.
- Standard Operating Procedures (SOPs): Integrating with SOPs to ensure that all data management processes align with QA standards.
68. How do eCRFs manage protocol amendments during an ongoing trial?
eCRFs manage protocol amendments by:
- Version Control: Implementing version control to manage changes to the eCRF that reflect protocol amendments.
- Training Updates: Providing training updates to site staff on new data entry requirements resulting from protocol amendments.
- Data Migration: Ensuring that existing data is correctly migrated to the new eCRF version without loss or errors.
- Audit Trail Documentation: Documenting all changes and the reasons for them in the audit trail to maintain transparency and compliance.
69. How do eCRFs support patient data confidentiality and compliance with data protection regulations like GDPR?
eCRFs support patient data confidentiality and compliance with data protection regulations through:
- Data Encryption: Encrypting data at rest and in transit to prevent unauthorized access.
- Anonymization and De-identification: De-identifying patient data to protect personal information while allowing for data analysis.
- Access Controls: Implementing role-based access controls to limit who can view or edit sensitive patient information.
- Consent Management: Managing and documenting patient consent in compliance with GDPR and other regulations, ensuring that data is only used for agreed purposes.
- Data Retention Policies: Establishing data retention policies that comply with regulatory requirements for how long patient data is stored.
70. How do eCRFs support the collection of patient-reported outcomes (PROs)?
eCRFs support the collection of patient-reported outcomes (PROs) by:
- ePRO Integration: Integrating with electronic Patient-Reported Outcomes (ePRO) systems, allowing patients to enter data directly into the eCRF via web portals or mobile apps.
- Real-time Data Capture: Capturing PRO data in real-time, enabling immediate access for study monitors and investigators.
- Customizable PRO Forms: Designing customizable forms specifically for PRO data, tailored to the specific outcomes being measured.
- Patient Engagement: Providing patients with an easy-to-use interface that encourages accurate and timely reporting of their experiences and outcomes.
71. What are the data management strategies for handling missing data in eCRFs?
Data management strategies for handling missing data in eCRFs include:
- Automated Alerts: Sending alerts to site staff when required data fields are missing, prompting them to complete the information.
- Query Resolution: Generating queries to resolve missing data issues by obtaining the correct information from the site or participant.
- Data Imputation: Using statistical methods to impute missing data, ensuring that analyses can proceed without bias.
- Documentation: Documenting all instances of missing data and the actions taken to address them, maintaining transparency and auditability.
72. How do eCRFs facilitate multi-language support in global clinical trials?
eCRFs facilitate multi-language support by:
- Localized Forms: Providing eCRF forms that are translated into multiple languages, ensuring that data can be collected consistently across different regions.
- Language Selection: Allowing users to select their preferred language for interacting with the eCRF system.
- Consistency Checks: Ensuring that translated forms maintain consistency with the original content, including validation rules and field definitions.
- Global Compliance: Complying with international regulations and standards for data collection in different languages, including local data protection laws.
73. How do eCRFs support the integration of imaging data in clinical trials?
eCRFs support the integration of imaging data by:
- Image Upload Capabilities: Allowing the direct upload of imaging data, such as X-rays, MRIs, or CT scans, into the eCRF.
- Metadata Capture: Capturing metadata related to the images, such as date, time, and imaging modality, alongside the clinical data.
- Viewer Integration: Integrating with imaging viewers that allow investigators to view and analyze images within the eCRF system.
- Data Linkage: Linking imaging data with other clinical data in the eCRF to enable comprehensive analysis and reporting.
74. What are the best practices for designing eCRF forms for complex clinical trials?
Best practices for designing eCRF forms for complex clinical trials include:
- User-Centered Design: Involving end-users in the design process to ensure that the forms are intuitive and easy to use.
- Modular Design: Breaking down the eCRF into modular components that can be easily modified or reused across different studies.
- Validation Checks: Incorporating comprehensive validation checks to ensure data accuracy and consistency.
- Customization: Allowing for the customization of forms to meet the specific needs of complex trials, including conditional logic and dynamic fields.
- Regulatory Alignment: Ensuring that the eCRF design complies with all relevant regulatory requirements, including data protection and audit trails.
75. How do eCRFs handle real-time data validation and error checking?
eCRFs handle real-time data validation and error checking by:
- Automatic Validation Rules: Implementing automatic validation rules that check data as it is entered, flagging any errors or inconsistencies immediately.
- Real-time Feedback: Providing real-time feedback to users, prompting them to correct errors before moving to the next section of the form.
- Consistency Checks: Running consistency checks between related data fields to ensure that entries are logical and coherent.
- Error Logs: Maintaining a log of all validation errors and how they were resolved, contributing to the audit trail and ensuring data quality.
Conclusion
Electronic Case Report Forms (eCRFs) have revolutionized the way clinical trials are conducted, offering a robust, efficient, and secure method for capturing and managing trial data. As the clinical research landscape continues to evolve, the adoption of eCRFs has become essential for ensuring data accuracy, compliance with regulatory requirements, and the overall success of clinical studies.
Through this comprehensive FAQ section, we’ve explored the various facets of eCRFs, from their fundamental benefits and functionalities to advanced features and best practices. Whether it’s ensuring data integrity through real-time validation, facilitating global trials with multi-language support, or integrating complex data sources like imaging and patient-reported outcomes, eCRFs provide a versatile platform that meets the diverse needs of modern clinical trials.
By embracing eCRFs, research teams can streamline their workflows, reduce the potential for errors, and focus on the critical aspects of clinical research—ultimately accelerating the development of new treatments and improving patient outcomes. As you continue to explore and implement eCRFs in your trials, the knowledge gained from these FAQs will serve as a valuable resource, guiding you through the complexities and helping you leverage the full potential of eCRFs.
The future of clinical trials lies in the effective use of digital tools like eCRFs, and by staying informed and adaptable, you can ensure that your research is not only compliant and efficient but also at the forefront of innovation in clinical research.


