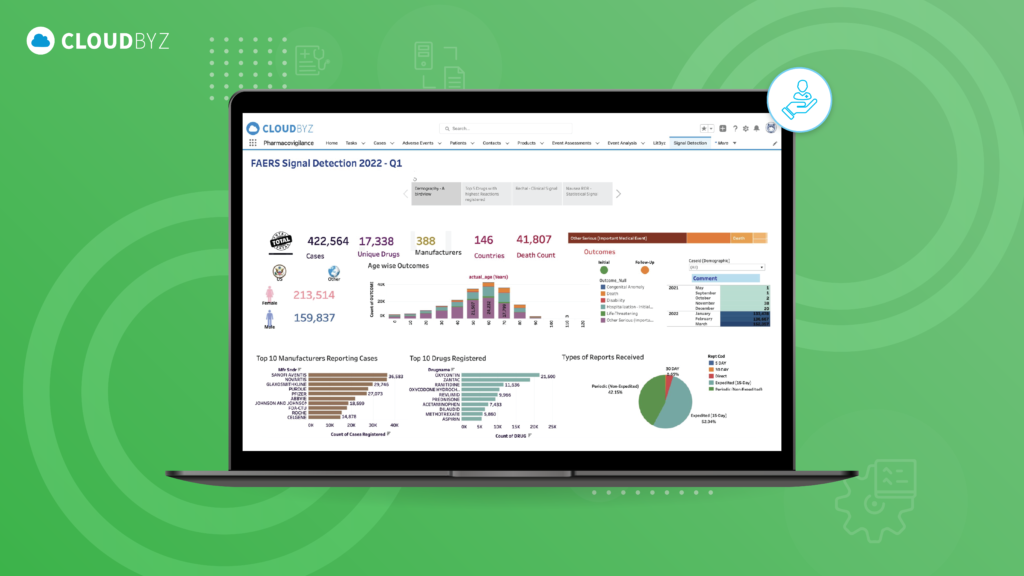
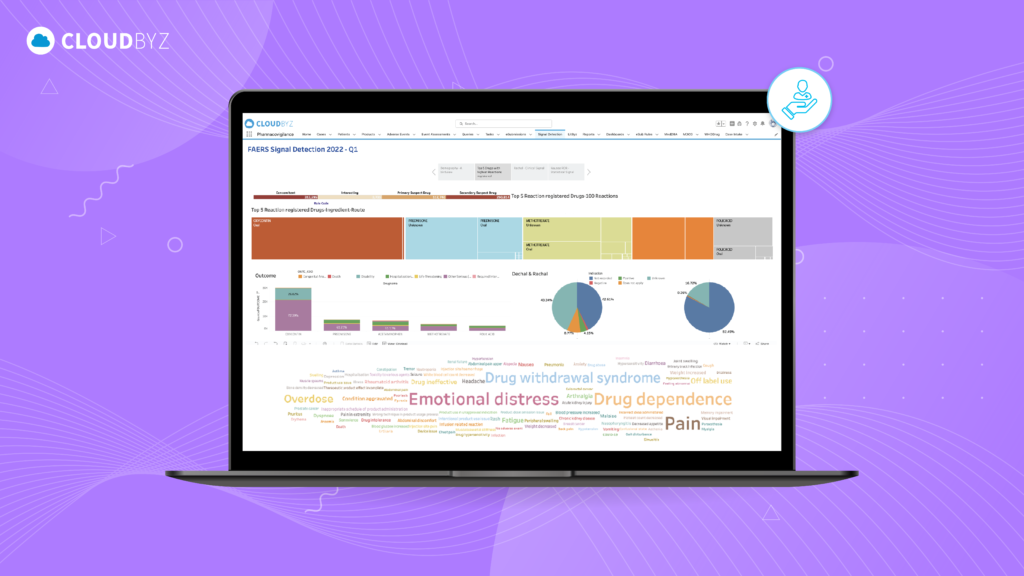
In the realm of pharmacovigilance, post-market surveillance (PMS) plays a crucial role in ensuring the safety and efficacy of pharmaceutical products once they are available to the general public. While pre-market clinical trials provide a controlled environment to assess a drug’s safety profile, real-world use can reveal new risks and adverse events that were not evident during clinical development. This stage of monitoring is critical for protecting public health, maintaining regulatory compliance, and sustaining the integrity of the pharmaceutical industry.
However, navigating the complexities of post-market surveillance presents several challenges, ranging from data collection and management to timely reporting and regulatory adherence. This article delves into these challenges and explores how Cloudbyz Safety & Pharmacovigilance offers a robust solution to streamline and enhance the PMS process.
The Challenges of Post-Market Surveillance in Pharmacovigilance
- Data Collection and Integration
- Challenge: In the post-market phase, adverse event data can be sourced from a wide range of channels, including healthcare providers, patients, social media, and electronic health records (EHRs). The diverse nature of these data sources complicates the collection, standardization, and integration of information necessary for accurate surveillance.
- Solution: Cloudbyz Safety & Pharmacovigilance is designed with advanced data integration capabilities, enabling seamless aggregation of adverse event data from various sources. Its flexible architecture supports real-time data collection, ensuring that all relevant information is captured and standardized for analysis, regardless of the data’s origin.
- Regulatory Compliance and Reporting
- Challenge: Regulatory bodies across the globe, such as the FDA, EMA, and MHRA, have stringent reporting requirements for adverse events. Non-compliance can lead to severe penalties, including product recalls and loss of market authorization. The challenge lies in staying up-to-date with ever-evolving regulations and ensuring that reports are timely, accurate, and complete.
- Solution: Cloudbyz Safety & Pharmacovigilance offers automated workflows that are continually updated to reflect the latest regulatory guidelines. The platform’s reporting tools are designed to generate and submit reports in the required formats, reducing the risk of human error and ensuring compliance with global regulatory standards.
- Continuous Monitoring and Signal Detection
- Challenge: The dynamic nature of post-market surveillance demands continuous monitoring of data to detect new safety signals promptly. Traditional methods of manual data review and analysis are time-consuming and may delay the identification of potential risks, putting patients at risk and companies in jeopardy of non-compliance.
- Solution: Cloudbyz leverages advanced analytics and artificial intelligence (AI) to facilitate continuous monitoring and signal detection. The platform’s AI-driven algorithms can identify patterns and correlations in vast datasets, enabling proactive identification of emerging safety concerns. This real-time monitoring capability allows for quicker decision-making and risk mitigation.
- Resource Constraints and Operational Efficiency
- Challenge: Managing post-market surveillance activities can be resource-intensive, requiring significant manpower and technological infrastructure. Smaller companies, in particular, may struggle with the operational burden, leading to inefficiencies and potential gaps in safety monitoring.
- Solution: Cloudbyz Safety & Pharmacovigilance is a cloud-based solution that offers scalability and flexibility. The platform’s automated processes reduce the need for manual intervention, freeing up valuable resources and enhancing operational efficiency. Furthermore, Cloudbyz’s user-friendly interface and comprehensive training modules make it accessible for companies of all sizes, enabling them to manage post-market surveillance effectively without overwhelming their teams.
- Globalization and Cross-Border Challenges
- Challenge: In an increasingly globalized market, pharmaceutical companies must navigate the complexities of cross-border regulatory requirements and adverse event reporting. Differences in regulatory expectations, language barriers, and data privacy concerns add layers of complexity to post-market surveillance.
- Solution: Cloudbyz is built with global compliance in mind, offering multi-language support and region-specific configurations. The platform ensures that adverse event reporting aligns with the regulatory frameworks of different regions, while its secure data management practices adhere to international data privacy standards, such as GDPR.
How Cloudbyz Ensures Continuous Monitoring and Reporting
Cloudbyz Safety & Pharmacovigilance stands out by providing a holistic approach to post-market surveillance. Its key features include:
- Real-Time Data Integration: The platform’s ability to aggregate and standardize data from various sources ensures that all relevant information is captured for continuous monitoring.
- Automated Workflows: By automating the reporting process, Cloudbyz ensures that reports are generated and submitted in a timely manner, adhering to the latest regulatory requirements.
- AI-Driven Signal Detection: Advanced analytics and AI tools within Cloudbyz enable proactive identification of potential risks, allowing companies to address safety concerns before they escalate.
- Scalability: Whether managing a single product or a diverse portfolio, Cloudbyz scales with your needs, offering a flexible solution that can grow with your company.
- Global Compliance: Cloudbyz’s global configurations ensure that your post-market surveillance activities remain compliant with international regulations, regardless of where your product is marketed.
Conclusion
Post-market surveillance is a critical component of pharmacovigilance, essential for ensuring the ongoing safety of pharmaceutical products after they enter the market. The challenges associated with PMS are significant, but with the right tools and strategies, they can be effectively managed. Cloudbyz Safety & Pharmacovigilance provides a comprehensive solution that addresses these challenges, offering advanced data integration, regulatory compliance, continuous monitoring, and global support. By leveraging Cloudbyz, companies can navigate the complexities of post-market surveillance with confidence, ensuring the safety and well-being of patients worldwide.


