
Let’s be real for a second—clinical research systems aren’t exactly everyone’s favorite topic but if you’re in the trenches of clinical research, you know they can make or break your day. So, let’s cut through the noise and talk about what’s really going on with these systems, why they’re driving everyone up the wall.
It’s 2024—Almost 2025 soon—and We’re Still Struggling with things like:
Reporting: Why Is It Such a Hassle?
Picture this: You’ve gathered a mountain of data from your ongoing clinical trial, and now you need to stay on top of it, making sense of the numbers and trends. But here’s the catch—your research system’s reporting tools feel like they belong in the Stone Age.
Sound Familiar?
Have you ever been stuck in these frustrating situations?
- Building or Modifying Reports: You need to create or tweak a report, but guess what? You can’t do it yourself. You have to bring in a developer or rely on your vendor’s team.
- Long Turnaround Times: Days, sometimes even weeks, or hey, even months go by before you finally get the reports you had requested and oh my my, if you happen to ask for an updated one.
- Juggling between Multiple tools: You’re forced to use tools like Power BI or Tableau just to view your metrics. Why are you tracking clinical trials in one system but then have to hop over to another just to see the results?
This kind of setup means you’re spending more time wrestling with your data than actually using it to make decisions. And let’s be honest—nobody signed up for that. A good research system should make reporting a breeze, not a battle.
Enter the Cloudbyz Way
Now, imagine this:
- You—yes, you—can build or modify reports using Cloudbyz’s intuitive drag-and-drop tool. No coding experience? No IT background? No problem.
- Seamless Integration: This tool is fully integrated into your CTMS system and embedded across your entire clinical research process.
- Total Control: Want to add a new metric to track in that report? Go for it! No need to wait on a team of developers or reach out to your vendor.
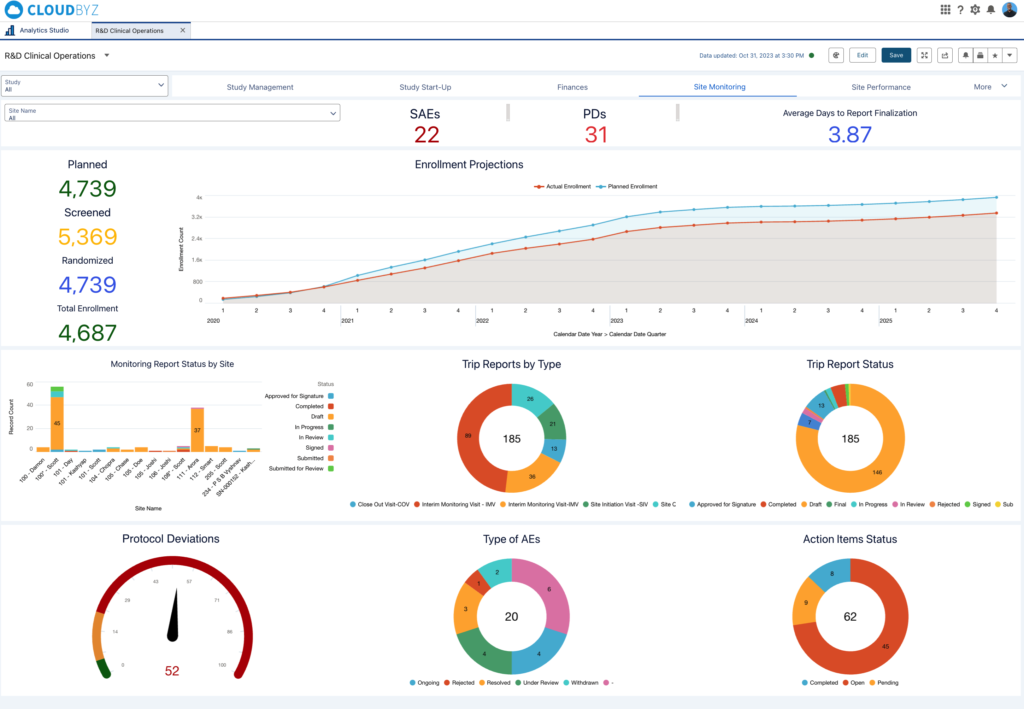
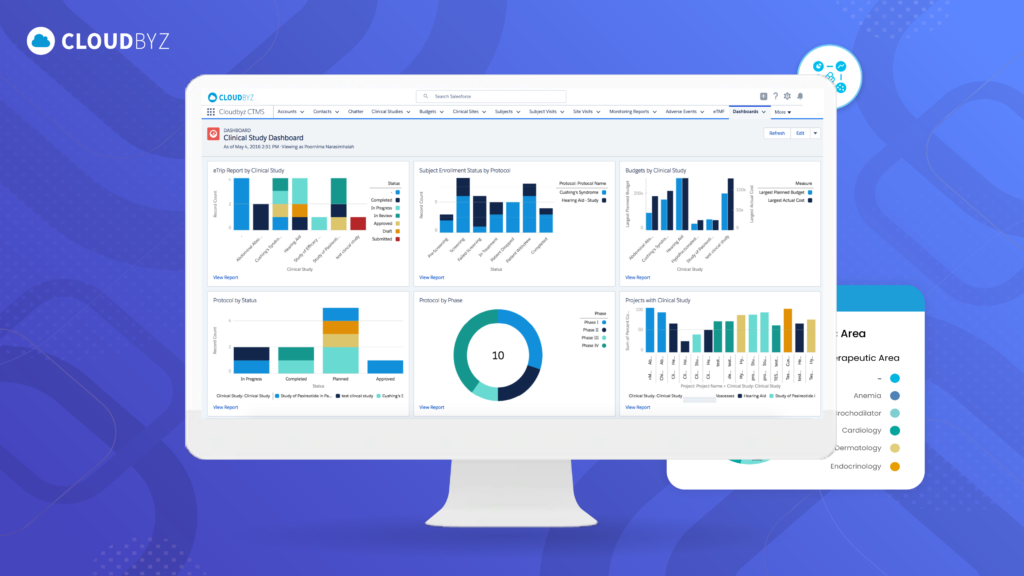
- Stunning Reports and Dashboards: And the best part? The reports and dashboards you’ll create aren’t just functional—they’re visually impressive and tailored to your exact needs.
See for Yourself:
The Juggling Act: Switching and Managing Multiple Systems?
Let’s talk about an interesting (relatable) scenario in clinical research: You’ve got one system for tracking where potential participants are in the recruitment funnel, another for managing all the activities and data within the trial, and another system for ensuring all your regulatory documents are up-to-date and audit-ready.
The problem, you ask? These systems don’t talk to each other. So, you’re stuck juggling them all, managing multiple logins and access control situations, hoping nothing important falls through the cracks—like making sure every participant’s consent form is properly logged and accessible when needed. It’s like trying to keep a bunch of spinning plates from crashing to the floor.
But here’s the reality—this kind of fragmented setup isn’t just annoying and tiring; it’s downright inefficient. Every time you switch from tracking participant engagement to updating trial data, and then to ensuring compliance with regulations, you’re losing valuable time and focus.
Still Not Convinced on This? No Problem, Let’s Keep Talking.
- Imagine you’re running a clinical trial, and a participant reports an adverse event. You start by entering the data into the EDC system.
- But that’s just the beginning of the headache. Next, you need to notify the Safety team, which means either manually re-entering the data into a completely separate Safety system or praying that the integration between these different systems doesn’t fail you.
- Then, you switch over to the CTMS to update the subject’s record, adjust timelines, and schedule follow-ups—all while juggling multiple platforms.
- But the chaos doesn’t stop there—you still need to upload the adverse event report into yet another system, the eTMF, making sure it’s properly stored for regulatory compliance.
- Throughout this entire process, you’re constantly bouncing between different systems, fighting with inconsistent data, and stressing over whether something critical got lost in translation.
Unified System a pipe dream? Enter the Cloudbyz Way
Imagine you’re conducting a clinical trial for a new drug.
A participant experiences an adverse event during a study visit, which is immediately recorded in the Cloudbyz EDC system.
*Instantly*
This data is automatically flagged in the Cloudbyz Safety module, prompting the safety team to assess the situation.
*Simultaneously*
The CTMS updates the participant’s profile and trial timeline, ensuring the event is logged and appropriate follow-ups are scheduled.
*In the meantime*
The corresponding documentation, including the adverse event report and regulatory communications, is instantly stored and organized within the eTMF, ensuring everything is compliant and ready for audit.
*And while all this happens in parallel*
With Cloudbyz, every step, from data capture to regulatory documentation, is seamlessly connected, minimizing risk and maximizing efficiency.
Imagine switching between your EDC and Safety with just a click within the same platform!!
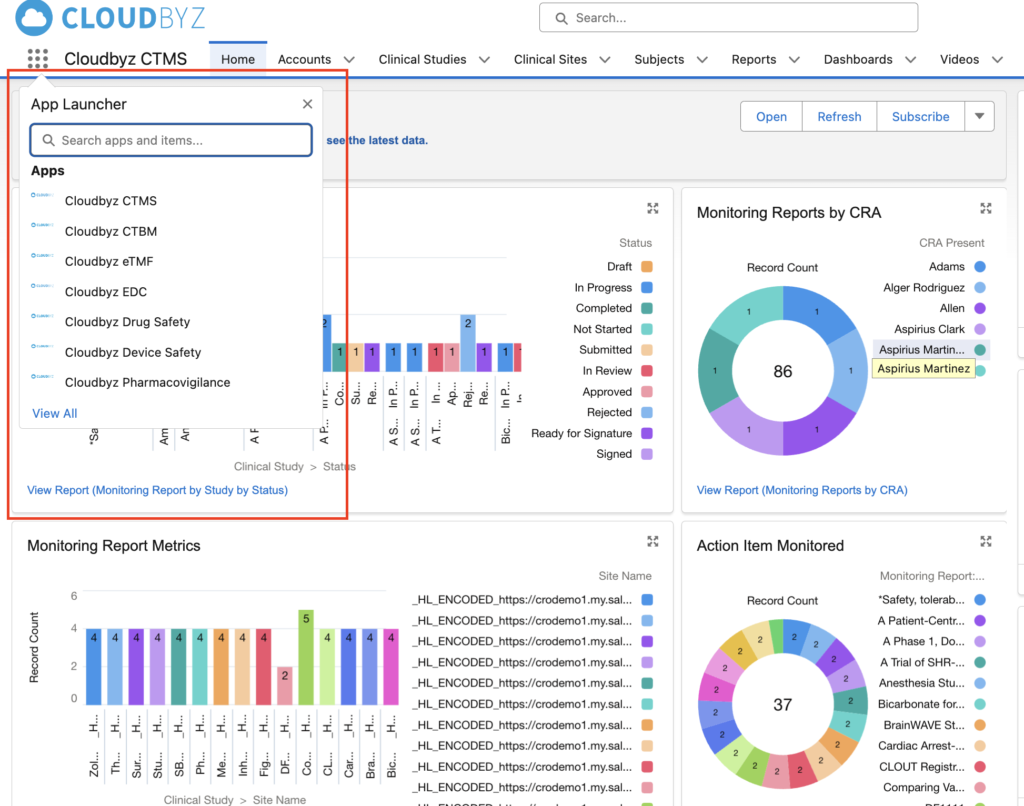
The Simplicity Factor: Why a User-Friendly System Matters
Let’s dive into a common challenge research teams face dealing with systems that feel more like a confusing mess than a useful tool.
Imagine this: Your team is in the midst of an important clinical trial, with timelines that are tight, and every detail matters. You log into your eClinical system, ready to make updates or analyze data, but instead of a smooth, efficient process, you’re met with a clunky interface that feels like it’s working against you.
Here’s what happens: Many eClinical platforms are burdened with overly complex menus and interfaces that require multiple steps just to perform a basic function. Users often find themselves digging through layers of options, losing precious time and momentum. What should be a simple task—like updating records, running reports, or tracking study progress—turns into a time-consuming chore that drains your focus and energy.
Why does this complexity hurt?
In clinical research, every second matters. When your team struggles with a complicated system, it not only slows down the workflow but also increases the chance of errors. A user-friendly eClinical system is designed to streamline your work, allowing you to focus on what’s truly important: conducting research, analyzing data, and making informed decisions.
Enter the Cloudbyz Way: Sleek, Intuitive, and Highly Functional!
Now, imagine if your system was as easy to use—intuitive, straightforward, and designed with the user in mind. You don’t have to scroll endless on the page to find information you need or struggle to find what you need.
What if you can simply find all the information about the study by simply switching tabs?
Check it out:
In a world where every dollar and every minute counts, having a system that’s simple to use isn’t just a nice-to-have—it’s a must-have.
Final Thoughts: Time for a Change?
If your clinical research system is driving you crazy, you’re not alone. From clunky reporting tools to fragmented workflows, these systems have a lot of room for improvement. But don’t settle for something that’s making your job harder. It might be time to explore new options and find a system that really works for you.

