You’re at the helm of a Phase I clinical trial—facing the challenge of managing a small sample size, dense data, and the immense responsibility of making decisions that could shape the future of a breakthrough drug. Now, take a moment and ask yourself, “Is my current system really equipped to handle this?”
A Typical Day in the Life
It’s Monday morning, or maybe even Tuesday after a well-deserved long weekend. You log into your clinical trial system, not just to check a few data points but to dive deep into a complex web of safety data, lab results, and protocol deviations—hallmarks of Phase I trials. Everything needs to be accurate, up-to-date, and at your fingertips.
Does This Sound Familiar?
If this resonates with your daily reality, you know how frustrating it can be when your system slows you down. Imagine, instead, having a system that does the heavy lifting for you, allowing you to focus on what really matters.
Key Checkpoints for Choosing an EDC System for early phase trials:
When evaluating an EDC system for your upcoming early phase trials, here are some critical questions you consider:

I’m sure this list goes on and on.
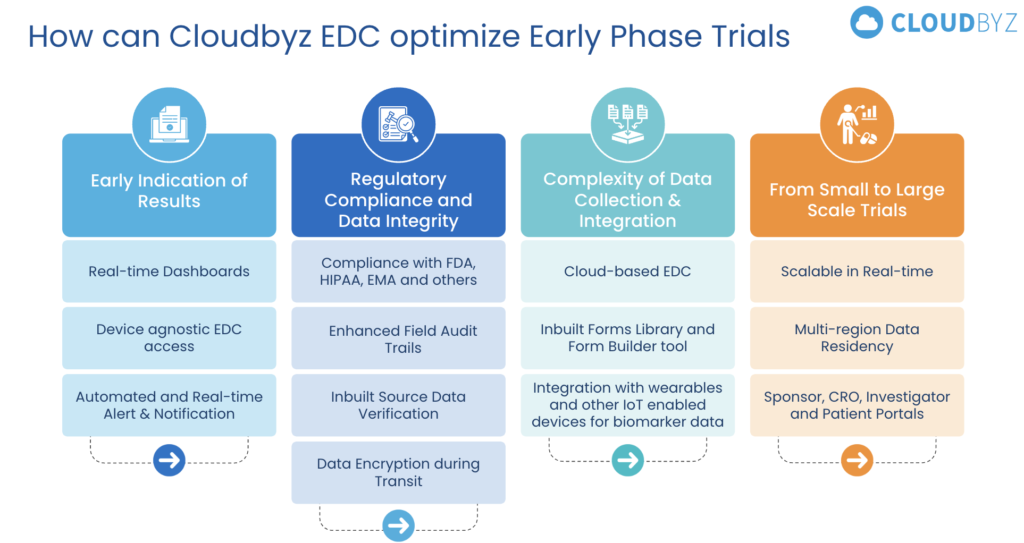
We recently held a webinar where we discussed how Cloudbyz EDC addresses the unique challenges of early-phase trials. Here are some key takeaways:
1) Real-Time Data Integration and Accessibility
The Challenge: Phase I trials demand seamless integration of diverse data sources to support real-time decision-making. Traditional systems often struggle with immediate access to critical safety, PK, and PD data, leading to potential delays.
Cloudbyz EDC’s Solution: Leveraging Salesforce’s robust API, Cloudbyz EDC integrates third-party data sources into a single, accessible platform. This ensures that all data, from lab results to real-time monitoring devices, is fully searchable, reportable, and available in real-time, enabling swift decision-making and enhanced trial oversight.
2) Integrated Safety Monitoring
The Challenge: Swift transitions between data capture and safety monitoring are critical in early-phase trials. Data silos in traditional systems can delay tracking adverse events and necessary interventions.
Cloudbyz’s Solution: Cloudbyz’s App Launcher enables seamless transitions between EDC and safety-related applications, ensuring safety data is consistently monitored and integrated, supporting rapid responses to emerging risks.
3) Configurable access management
The Challenge: Phase I trials involve multidisciplinary teams such as CRAs, Clinical Monitors, and Data Managers each requiring tailored access to specific datasets without being overwhelmed by irrelevant data.
Cloudbyz EDC’s Solution: Cloudbyz EDC offers a user-friendly interface that allows you to tailor access based on user roles. Any information or reports within the system, including dashboards and widgets on your home page, can be configured per role, ensuring that each team member accesses the exact data they need, enhancing efficiency and focus.
4) Gaining Real-Time Insights with Comprehensive Data Analysis
The Challenge: Early-phase trials require continuous analysis of complex data, such as PK/PD profiles and metrics. Real-time insights are critical for informed decision-making.
Cloudbyz EDC’s Solution: The Study Data Overview in Cloudbyz EDC offers real-time insights across your trial, allowing you to drill down from study overviews to specific data points like sites, subjects, and visits. The Study Overview tab provides essential details, while Planning covers study teams, visit plans, and protocol tracking. Team Access controls who sees what, ensuring secure data handling. The Visit Plan accommodates multiple study arms and protocol versions. The List View shows subject statuses and queries, while the Query Management module filters and tracks open queries, ensuring timely resolutions.
5) Unique Study Designs in Early-Phase Trials
The Challenge: No two clinical trials are the same, particularly in early-phase studies where each protocol has unique requirements.
Cloudbyz EDC’s Solution: The Unique Study Setup feature includes all necessary form-building components and study-specific parameters tailored for each type of study. Whether you need to configure the Subject Number Schema, set up a Visit Plan, or define Data and Matrices statuses, the system provides the flexibility to customize these elements to fit your study’s exact needs.
6) Capturing Diverse Data Needs
The Challenge: Early-phase trials involve capturing diverse data types across various clinical activities. The challenge lies in managing this data efficiently without compromising on accuracy or system performance.
Cloudbyz EDC’s Solution: Cloudbyz EDC’s user-friendly and no-code form builder features, including eCRF Builder, eConsent Builder, and others, enable anyone on your team to create and customize forms without any IT experience. This empowers your team to adapt quickly to the evolving needs of your trial. What sets Cloudbyz EDC apart is its ability to handle over 25+ data types, allowing you to capture a wide range of data precisely as required by your study. The system also includes advanced configuration options, such as Setup Rules, Successor questions and sections, Data Variables, and Permissions, giving you full control over how data is collected, validated, and accessed. This level of flexibility ensures that all relevant data is captured accurately.
7) Adapting to Protocol Changes
The Challenge: Implementing protocol amendments requires swift updates to forms and data collection tools. Delays or errors can lead to inconsistencies and protocol deviations.
Cloudbyz EDC’s Solution: The Form Versioning and Upgrades feature allows rapid implementation of protocol changes, ensuring consistency with the latest requirements. You can preview forms as different roles, manage multiple versions with effective dates and statuses, and easily upgrade or downgrade forms across your study. This agility is crucial in early-phase trials, where protocols evolve rapidly, ensuring your data collection remains accurate and aligned with evolving study needs.
8) Ensuring Scalability and Consistency Across Phases
The Challenge: Maintaining consistency in data collection across different trial phases is crucial. Variations can lead to discrepancies and regulatory challenges.
Cloudbyz EDC’s Solution: The Forms Library in Cloudbyz EDC offers a centralized repository for storing, managing, and reusing forms across studies and phases. This is essential in early-phase trials where intensive and varied data collection occurs. As you move from Phase I to later phases, the ability to reuse and adapt forms ensures consistent data collection methods, aiding in regulatory compliance and data comparability across all trial phases.
9) Efficient Data Export and Real-Time Analysis
The Challenge: Early-phase trials require rapid data export and analysis to inform ongoing decisions, such as dose adjustments and safety monitoring.
Cloudbyz EDC’s Solution: The Form Export feature allows targeted, efficient data export based on specific criteria like Study, Region, Site, Study Arm, and Subject. You can also filter by Subject Visit and Form Status, ensuring you extract exactly what you need for timely analysis. This capability is crucial in early-phase trials, where quick access to specific data enables interim analysis and informed decision-making, facilitating rapid adjustments during critical trial phases.
Final Thoughts
Phase I clinical trials are critical to drug development, and the data captured during these trials can make or break a drug’s future. Traditional data capture methods are increasingly inadequate to meet the demands of modern trials. Cloudbyz EDC offers the automation, integration, and real-time monitoring necessary to ensure these trials are successful, safe, and efficient.
If your trial is still relying on outdated methods, it’s time to consider the benefits of an EDC system like Cloudbyz. It not only streamlines operations and reduces costs but also enhances data integrity and participant safety—two elements that are non-negotiable in the world of clinical trials.

