Ensuring Compliance in Consumer Product Testing: Challenges and Solutions
Consumer product testing is a critical process for companies that

Dinesh is a distinguished visionary leader with more than 20 years of experience in the IT industry. He has an impressive track record in strategy and management consulting, business & IT transformation, innovative digital platform implementations, building practice and capabilities in new niche markets. Prior to founding Cloudbyz, Dinesh was with HCL where he led a multi-million dollar business. You can find Dinesh on LinkedIn.

Consumer product testing is a critical process for companies that

In today’s fast-paced and competitive market, consumer product companies are
The adoption of unified eClinical platforms is poised to transform the landscape of clinical operations. By breaking down the silos that have traditionally hampered trial efficiency and data integrity, these platforms offer a more cohesive, transparent, and agile approach to managing clinical trials. As the life sciences industry continues to innovate and evolve, the unified eClinical platform will play a pivotal role in enabling organizations to conduct more efficient, compliant, and successful trials.
In the rapidly evolving landscape of clinical research, efficiency and
In an industry where the integrity and accuracy of clinical trial documentation are paramount, Cloudbyz offers a comprehensive and innovative approach to eTMF management. By integrating Cloudbyz eTMF with AI-powered solutions like ClinRedact and ClinExtract, organizations can streamline their document management processes, enhance compliance, and drive greater efficiency across their clinical operations.

For life sciences companies looking to stay ahead of the curve, Cloudbyz represents the future of drug safety management. Whether you are a pharmaceutical giant, a biotech innovator, or a medical device manufacturer, Cloudbyz Safety & PV solutions can help you navigate the complexities of modern pharmacovigilance with confidence and ease.
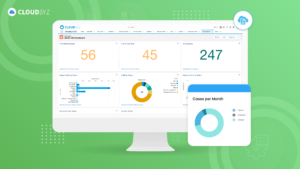
Implementing an EDC system in a biotech company can significantly enhance clinical trial efficiency, data accuracy, and overall productivity. By following best practices, such as defining clear objectives, selecting the right system, ensuring regulatory compliance, and providing adequate training, you can overcome common challenges and achieve a successful implementation. Continuous monitoring and evaluation will help maintain system performance and drive ongoing improvements, ensuring your EDC system remains a valuable asset in your clinical research endeavors.
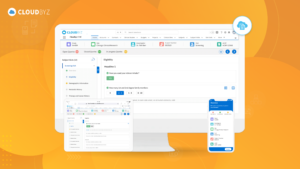
Cloudbyz EDC offers powerful integration capabilities with various systems and technologies, enabling efficient data management and enhanced trial operations. By following best practices for managing and securing clinical trial data, organizations can ensure data integrity, compliance, and security, ultimately contributing to the success of their clinical trials. Cloudbyz EDC provides the tools and features necessary to navigate the complexities of clinical trial data management, making it an ideal choice for modern clinical research.
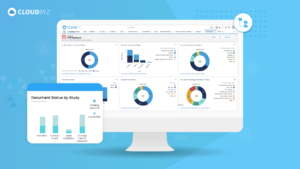
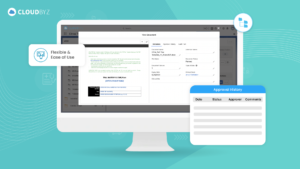
In the complex and highly regulated world of clinical research, essential documents serve as the foundation upon which successful and trustworthy clinical trials are built. Their meticulous management and organization are key to the smooth execution and ultimate success of any clinical study. Implementing best practices for managing these documents ensures that all regulatory and ethical standards are met, protecting the rights and safety of participants and supporting the validity of the study outcomes.
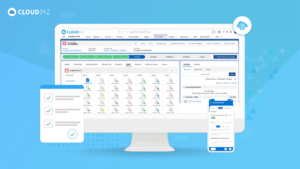
An Electronic Data Capture (EDC) solution built natively on Salesforce offers a powerful tool for accelerating clinical studies. By enhancing data accuracy, improving efficiency, facilitating collaboration, and ensuring regulatory compliance, Cloudbyz EDC can drive faster, more reliable study outcomes. As the clinical research landscape continues to evolve, leveraging advanced technologies like Cloudbyz EDC will be essential for staying competitive and achieving success in clinical trials.
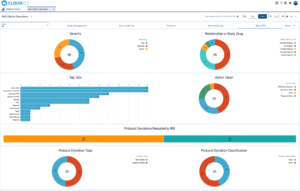
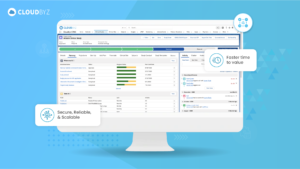
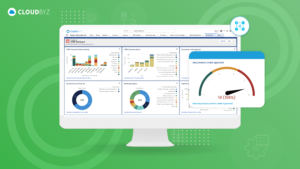
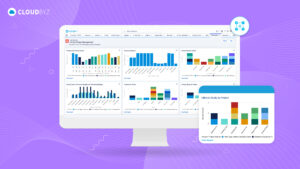
For senior clinical operations executives in pharma, biotech, medical devices, diagnostics, and CROs, real-time visibility into clinical trial metrics is a game-changer. Cloudbyz eClinical solution, built on the Salesforce platform, provides the comprehensive, real-time insights needed to manage complex clinical operations effectively. By leveraging Cloudbyz’s robust dashboards and integrated platform, organizations can enhance their clinical trial management, ensure compliance, and ultimately bring new therapies to market faster.

Diagnostics are the cornerstone of innovation in biomarker-based precision medicine and cell and gene therapy. By enabling the identification, selection, and monitoring of patients, diagnostics enhance the precision and effectiveness of these advanced therapies. Moreover, in post-market care, diagnostics ensure the continued safety and success of treatments, contributing to better health outcomes for patients. As diagnostic technologies continue to evolve, they will undoubtedly unlock new possibilities in personalized medicine, transforming the future of healthcare. The integration of AI and machine learning, along with advancements in immunotherapy and regenerative medicine, will further expand the horizons of what diagnostics can achieve, heralding a new era of medical excellence. Furthermore, the acceleration of IVD trials is crucial to making these diagnostic tools available more rapidly, thereby supporting the timely and efficient implementation of innovative therapies and ultimately improving patient care worldwide.
At Cloudbyz, our mission is to empower our clients to achieve their business goals by delivering innovative, scalable, and intuitive cloud-based solutions that enable them to streamline their operations, maximize efficiency, and drive growth. We strive to be a trusted partner, dedicated to providing exceptional service, exceptional products, and unparalleled support, while fostering a culture of innovation, collaboration, and excellence in everything we do.
ISO 9001:2015 and ISO 27001:2013 Certified