Streamlining Clinical Trials: Common Workflow Automations in Electronic Trial Master File (eTMF) Systems
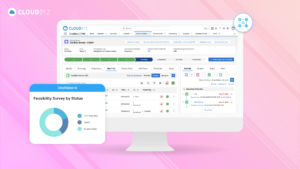
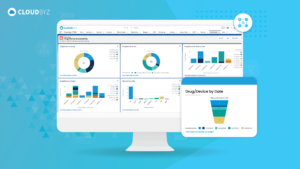
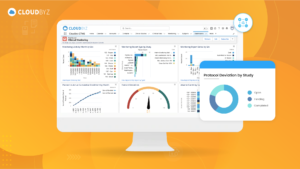
Electronic Trial Master File (eTMF) systems with workflow automations are indispensable tools for efficiently managing the complex documentation associated with clinical trials. These systems not only streamline processes but also enhance compliance, transparency, and collaboration among trial stakeholders. As the healthcare and pharmaceutical industries continue to evolve, eTMF systems with workflow automations play a crucial role in expediting the development and delivery of new treatments to patients while maintaining the highest standards of quality and compliance.