
Transforming Challenges into Opportunities: Success Story
The customer is a large multinational pharmaceutical company focused on

Dinesh is a distinguished visionary leader with more than 20 years of experience in the IT industry. He has an impressive track record in strategy and management consulting, business & IT transformation, innovative digital platform implementations, building practice and capabilities in new niche markets. Prior to founding Cloudbyz, Dinesh was with HCL where he led a multi-million dollar business. You can find Dinesh on LinkedIn.

The customer is a large multinational pharmaceutical company focused on
CDMs play a crucial role in ensuring that data collected from various sources is complete, accurate, and consistent across all sources. They need to have a thorough understanding of the technology used in DCTs and develop processes to ensure data quality and integrity. By doing so, they can help to ensure the success of decentralized clinical trials and provide reliable data for analysis and reporting.

The customer is a fully integrated clinical research organization (CRO)

The customer is a leading genetic testing company that operates

The customer is one of Australia’s largest CRO with a

The customer is a leading multinational pharmaceutical company based in
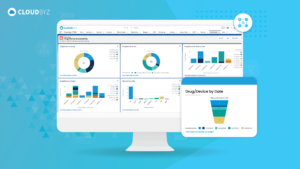
Unified clinical trial platform can help accelerate the launch of medical devices by streamlining data management, enhancing collaboration, automating manual tasks, improving compliance, and enabling faster study completion. By providing real-time access to data and enabling faster decision-making, a unified platform helps ensure that medical devices reach the market faster, improving patient access to innovative treatments.
The MDCG regulatory reporting guidelines are an essential part of the EU’s regulatory framework for medical devices. By ensuring that manufacturers report on adverse events, corrective actions, and ongoing safety data, the guidelines help to ensure the safety and efficacy of medical devices used by patients in the EU. It is imperative that manufacturers comply with these guidelines to ensure that their devices can continue to be sold in the EU market and to build trust with the public.

Mid-size Clinical Research Organizations (CROs) play a significant role in the pharmaceutical and biotech industry by providing various services such as study design, clinical trial management, data management, and biostatistics. However, mid-size CROs face unique challenges in offering differentiated services and value to their customers to stay competitive against larger CROs.

Discover how a pharmacovigilance solution built on the Salesforce platform can streamline adverse event reporting, enhance patient safety, and improve compliance.

pharmacovigilance medical coding automation is a promising and rapidly evolving field that has the potential to revolutionize the way pharmacovigilance professionals monitor and assess medication safety. By leveraging the power of AI and machine learning, pharmacovigilance organizations can streamline the medical coding process, improve the accuracy and consistency of ADR reporting, and gain new insights into medication safety trends and risks.

Clinical trials are complex and resource-intensive endeavors. Therefore, there is
At Cloudbyz, our mission is to empower our clients to achieve their business goals by delivering innovative, scalable, and intuitive cloud-based solutions that enable them to streamline their operations, maximize efficiency, and drive growth. We strive to be a trusted partner, dedicated to providing exceptional service, exceptional products, and unparalleled support, while fostering a culture of innovation, collaboration, and excellence in everything we do.
ISO 9001:2015 and ISO 27001:2013 Certified