In clinical research operations, real-time visibility into operational metrics is crucial for success. Senior clinical operations executives in pharma, biotech, medical devices, diagnostics, and CROs require comprehensive dashboards that provide actionable insights to make informed decisions. Cloudbyz’s eClinical solution, built on the robust Salesforce platform, offers a transformative approach to managing clinical operations metrics and key performance indicators (KPIs).
The Power of Real-Time Metrics in Clinical Operations
Clinical operations involve managing multiple complex processes, from study planning and site monitoring to patient enrollment and financial tracking. The ability to access and analyze real-time data is essential to ensure these processes run smoothly and efficiently. Here are some of the critical metrics and KPIs that Cloudbyz eClinical solution enables for real-time visibility:
- Studies by Phase: Understanding the distribution of studies across different phases (Phase I-IV) helps in resource allocation and strategic planning. Cloudbyz provides a visual representation of ongoing studies, allowing executives to quickly assess progress and identify potential bottlenecks.
- Studies by Therapeutic Area: This metric provides insights into the focus areas of the clinical trials, helping organizations align their efforts with strategic priorities. Cloudbyz’s dashboards allow for easy tracking of studies by therapeutic areas, ensuring balanced portfolio management.
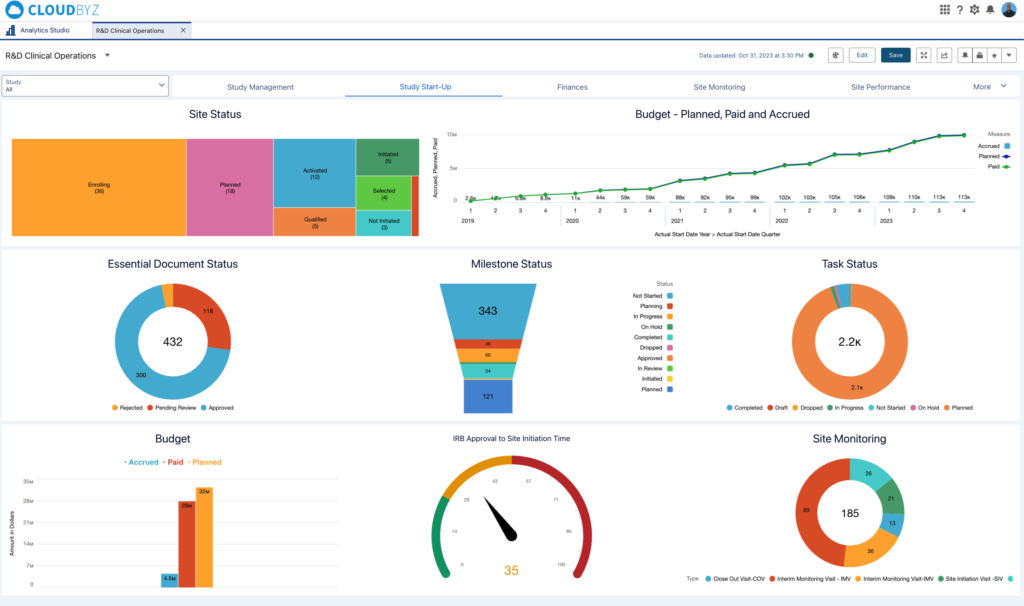
- Study Status: The status of each study, whether it’s in feasibility, planning, start-up, enrolling, in progress, or submitted for review, is critical for timeline management. Cloudbyz enables real-time tracking of study status, facilitating proactive management and timely interventions.
- Enrollments by Site: Monitoring patient enrollments across various sites is essential for ensuring study timelines are met. Cloudbyz’s solution offers detailed enrollment data, highlighting the performance of each site and enabling targeted support to underperforming sites.
- Site Monitoring Visits: Effective site monitoring is key to maintaining compliance and data integrity. Cloudbyz provides a comprehensive view of monitoring visits, including close-out visits, interim monitoring visits, and site initiation visits, ensuring thorough oversight.
- Budget and Finances: Financial tracking is a critical aspect of clinical operations. Cloudbyz’s financial dashboards provide real-time visibility into accrued, paid, and planned budgets, helping executives manage costs and avoid budget overruns.
- Essential Document Status: Ensuring that all essential documents are approved and up-to-date is crucial for regulatory compliance. Cloudbyz enables tracking of document status, from pending review to approval, ensuring no document is overlooked.
- Task and Milestone Status: Tracking tasks and milestones helps keep the study on schedule. Cloudbyz’s solution offers a detailed view of task completion and milestone achievement, allowing for timely adjustments to the project plan.
- Protocol Deviations: Monitoring protocol deviations is essential for maintaining study integrity. Cloudbyz provides real-time data on protocol deviations, categorized by type and severity, enabling swift corrective actions.
- Adverse Events (AEs): Tracking and managing adverse events is critical for patient safety. Cloudbyz offers detailed dashboards on AEs, including severity, relationship to study drug, and actions taken, ensuring comprehensive safety monitoring.
Deep Dive into Cloudbyz eClinical Solution: Transforming Clinical Operations
Enhanced Study Management
Managing a clinical study involves a multitude of moving parts, from initial planning to final review. Cloudbyz eClinical solution excels in providing real-time insights into each phase of the study, ensuring nothing falls through the cracks.
- Detailed Phase Tracking: By visualizing studies by phase, Cloudbyz enables executives to see the exact distribution of their clinical trials across Phase I to Phase IV. This helps in predicting resource needs and aligning operational strategies with developmental timelines.
- Therapeutic Area Insights: Understanding the therapeutic focus of ongoing studies can aid in portfolio management and strategic alignment. Cloudbyz’s dashboards offer a clear view of therapeutic areas, allowing companies to focus their resources on the most promising fields.
Comprehensive Study Status Monitoring
Keeping track of the status of each study is crucial for timely decision-making. Cloudbyz provides a holistic view of study progress, making it easier to identify and address issues early on.
- Feasibility to Submission: From feasibility to submission for review, every stage of a clinical study is meticulously tracked. This ensures that all processes are transparent and any delays can be promptly managed.
- Enrollment and Site Performance: Detailed enrollment metrics across sites highlight the performance of each location, helping to identify high-performing sites and those that need additional support.
Financial Oversight and Budget Management
Financial management is a critical aspect of clinical operations. Cloudbyz offers robust financial tracking tools to ensure budgets are adhered to and financial goals are met.
- Accrued, Paid, and Planned Budgets: Real-time tracking of accrued, paid, and planned budgets helps in financial forecasting and ensures that there are no surprises at the end of the fiscal period.
- Cumulative Financial Metrics: By tracking cumulative planned and paid budgets over time, Cloudbyz provides a long-term view of financial health, allowing for more strategic financial planning.
Document and Task Management
Ensuring that all necessary documents are in order and tasks are completed on time is essential for regulatory compliance and operational efficiency.
- Essential Document Status: Cloudbyz tracks the status of essential documents, from pending review to approval, ensuring regulatory compliance and audit readiness.
- Task Completion and Milestones: Detailed tracking of task status and milestone achievements helps keep the study on schedule. Cloudbyz’s dashboards provide a clear view of pending tasks and upcoming milestones.
Protocol Deviations and Adverse Event Management
Maintaining the integrity of a clinical trial requires diligent monitoring of protocol deviations and adverse events. Cloudbyz provides tools to track and manage these critical aspects.
- Protocol Deviations: By categorizing protocol deviations by type and severity, Cloudbyz ensures that any deviations are promptly addressed and corrective actions are implemented.
- Adverse Events Tracking: Detailed dashboards on adverse events, including severity and relationship to the study drug, help in maintaining patient safety and ensuring comprehensive monitoring.
Site Monitoring and Performance
Effective site monitoring is crucial for maintaining data integrity and compliance. Cloudbyz offers tools to ensure thorough oversight of site performance.
- Monitoring Visits: Detailed tracking of site monitoring visits, including close-out visits, interim monitoring visits, and site initiation visits, ensures comprehensive oversight.
- Site Performance Metrics: Cloudbyz provides insights into site performance, helping to identify and address issues promptly.
How Cloudbyz eClinical Solution Empowers Clinical Operations
Cloudbyz eClinical solution, built on the Salesforce platform, offers several advantages that empower clinical operations:
- Integrated Platform: Cloudbyz integrates all aspects of clinical trial management into a single platform, providing a unified view of operations. This integration eliminates data silos and ensures consistency across different functions.
- Real-Time Data: With Cloudbyz, clinical operations executives have access to real-time data, enabling immediate insights and quicker decision-making. This real-time visibility is crucial for maintaining study timelines and addressing issues proactively.
- Customizable Dashboards: The solution offers customizable dashboards that cater to the specific needs of different stakeholders. Executives can tailor their view to focus on the metrics that matter most to them.
- Scalability and Flexibility: Built on Salesforce, Cloudbyz offers the scalability to handle large volumes of data and the flexibility to adapt to the unique requirements of different clinical trials.
- Compliance and Security: Cloudbyz ensures compliance with industry regulations and provides robust security features to protect sensitive clinical data.
Conclusion
For senior clinical operations executives in pharma, biotech, medical devices, diagnostics, and CROs, real-time visibility into clinical trial metrics is a game-changer. Cloudbyz eClinical solution, built on the Salesforce platform, provides the comprehensive, real-time insights needed to manage complex clinical operations effectively. By leveraging Cloudbyz’s robust dashboards and integrated platform, organizations can enhance their clinical trial management, ensure compliance, and ultimately bring new therapies to market faster.
Cloudbyz’s commitment to innovation and excellence in clinical trial management makes it the ideal partner for organizations seeking to optimize their clinical operations. Embrace the power of real-time metrics with Cloudbyz and transform your clinical trial management today.
For more information about Cloudbyz eClinical solutions, visit Cloudbyz. Connect with us on LinkedIn.


