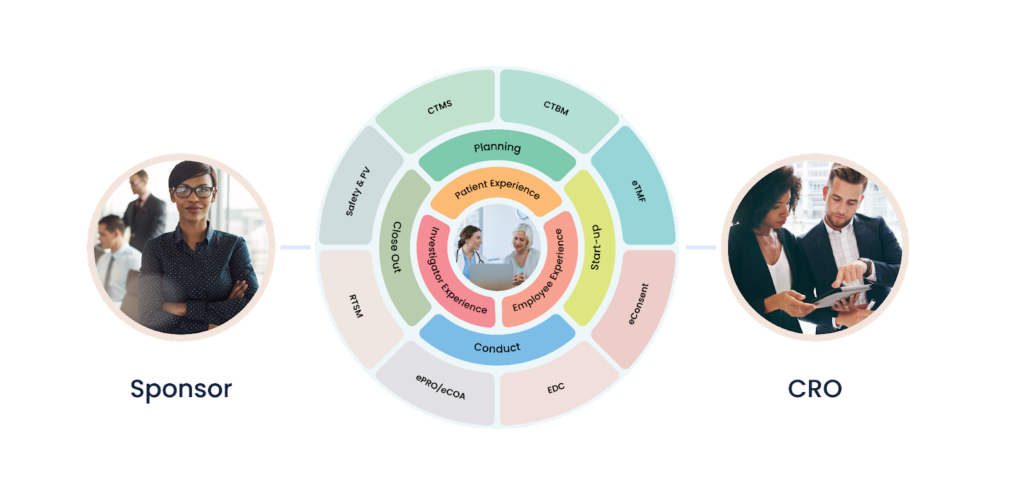
Clinical trials are the foundation of medical advancements, providing essential data for the development of new treatments and therapies. Sponsors and Contract Research Organizations (CROs) play a crucial role in this process, coordinating and overseeing the clinical research conducted at various sites. However, clinical site engagement is not without its challenges. In this blog, we will explore the major obstacles faced by sponsors and CROs in clinical site engagement and propose strategies for overcoming these hurdles.
- Site Identification and Selection:
One of the first challenges in clinical site engagement is identifying and selecting suitable sites for a trial. Sponsors and CROs must ensure that chosen sites have the appropriate facilities, personnel, and patient populations to meet the study’s requirements. This can be a time-consuming process, involving extensive research and evaluation.
Strategies to overcome this challenge include:
- Utilizing site networks and databases to identify potential sites
- Conducting thorough feasibility assessments to ensure sites meet study criteria
- Engaging with sites early on to foster a collaborative relationship
- Site Activation and Start-up Delays:
Once a site is selected, it must be activated and prepared for the study. This process involves obtaining regulatory approvals, negotiating contracts, and setting up the necessary infrastructure. Delays in site activation can result in increased costs and prolonged timelines for a clinical trial.
Strategies to overcome this challenge include:
- Streamlining the site activation process through effective project management and communication
- Implementing a standardized approach to contracts and budgets to minimize negotiation time
- Providing comprehensive training to site staff to facilitate efficient study start-up
- Patient Recruitment and Retention:
Patient recruitment and retention are critical to the success of a clinical trial. However, sponsors and CROs often face difficulties in identifying eligible patients and keeping them engaged throughout the study.
Strategies to overcome this challenge include:
- Developing targeted recruitment strategies that consider factors such as disease prevalence and patient demographics
- Utilizing digital platforms and social media for patient outreach
- Enhancing patient engagement through frequent communication, education, and support
- Data Quality and Compliance:
Ensuring data quality and compliance with regulatory standards is a top priority for sponsors and CROs. Discrepancies or non-compliance can compromise the integrity of a clinical trial and result in regulatory penalties.
Strategies to overcome this challenge include:
- Implementing robust data management systems and processes to ensure data accuracy and consistency
- Conducting regular monitoring and audits to identify and address any issues
- Providing ongoing training and support to site staff to maintain compliance
- Communication and Collaboration:
Effective communication and collaboration between sponsors, CROs, and clinical sites are essential for successful clinical trial execution. However, with multiple stakeholders involved, maintaining clear and consistent communication can be a challenge.
Strategies to overcome this challenge include:
- Establishing a single point of contact for each site to streamline communication
- Utilizing technology and communication platforms to facilitate collaboration and information sharing
- Holding regular meetings and updates to ensure all parties are informed and engaged
Conclusion:
Sponsors and CROs face numerous challenges in clinical site engagement, from site selection and activation to patient recruitment and data quality. By implementing targeted strategies and leveraging technology, these organizations can overcome these hurdles and pave the way for more efficient and successful clinical trials. As the industry continues to evolve, sponsors and CROs must remain adaptable and innovative to keep pace with the ever-changing landscape of clinical research.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com


