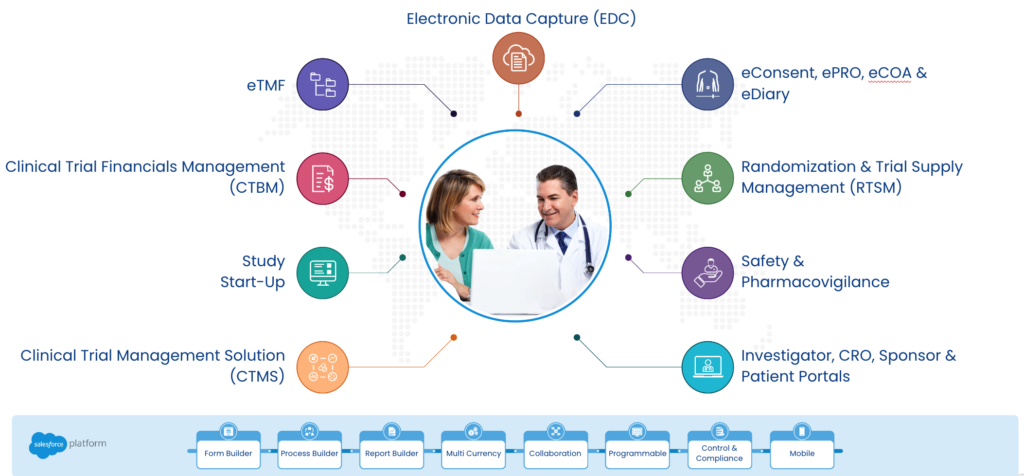
The landscape of clinical trial management is undergoing a significant transformation, driven by the complex demands of modern medical research. At the heart of this change is a move away from traditional point systems—discrete tools designed for specific tasks within a trial—to comprehensive platform solutions that offer a unified approach to managing all aspects of a trial. This shift is not merely technological but represents a fundamental rethinking of how clinical trials are conducted, promising to enhance efficiency, compliance, and the overall quality of research outcomes. As we delve into the challenges posed by point systems, it becomes clear that a platform approach is not just beneficial but essential for the future of clinical trials.
Challenges of Point Systems in Clinical Trial Management
1. Silos of Information:
Point systems, by design, focus on specific functions within the clinical trial process, such as patient recruitment, data collection, or inventory management. This specialization leads to the creation of information silos, where data is trapped within specific systems and difficult to aggregate or analyze in a cohesive manner. The consequences are manifold: researchers may struggle to gain a holistic view of trial progress, critical data insights might be overlooked, and the risk of data inconsistencies increases, potentially compromising the integrity of the trial. Moreover, the effort to manually integrate data from these silos can be prohibitively time-consuming and prone to human error, leading to delays and increased costs.
Furthermore, these silos hinder collaboration and communication among stakeholders. For instance, a clinician may not have immediate access to patient recruitment statuses, or a trial manager might lack real-time visibility into drug inventory levels. This compartmentalization of information creates barriers to efficient trial management and can delay response times to critical issues, impacting trial timelines and outcomes.
2. Scalability and Flexibility:
The clinical trial landscape is rapidly evolving, with trials becoming more complex and global in scope. Point systems, however, lack the inherent flexibility to adapt to changing trial designs, regulatory environments, and technological advancements. This rigidity means that as trials scale up or as new needs emerge, these systems can quickly become obsolete or require significant modifications, which are often costly and time-intensive to implement.
Moreover, the integration of new technologies or methodologies into existing point systems can be a cumbersome process. For example, incorporating digital health technologies, such as wearables for remote monitoring, into a legacy data collection system can present significant technical challenges. This lack of adaptability not only limits the potential for innovation within trials but also can extend timelines and inflate budgets, as additional resources are devoted to system modifications and workarounds.
3. User Experience and Accessibility:
The fragmented nature of point systems creates a disjointed user experience for all stakeholders involved in a clinical trial. Participants may have to navigate through multiple platforms for different phases of the trial, while researchers and sponsors juggle various interfaces to access the data and insights they need. This not only complicates training and increases the likelihood of user errors but also can hinder participant engagement and satisfaction.
Accessibility issues arise as well, with critical data and functionalities spread across different systems. Stakeholders may find it challenging to access the comprehensive insights needed for informed decision-making, potentially leading to delays or suboptimal outcomes. For global trials, the situation is compounded by the need to accommodate diverse regulatory and linguistic requirements, further stretching the capabilities of point systems.
4. Compliance and Security:
Ensuring compliance with ever-changing regulatory guidelines is a critical concern in clinical trial management. Point systems complicate this task by requiring separate updates and validations to meet new regulations, a process that is both time-consuming and prone to oversights. The disjointed nature of these systems also poses risks to data integrity and auditability, critical factors in regulatory compliance.
Security concerns are equally paramount, as clinical trials deal with sensitive participant data that must be protected against breaches. Point systems, with their multiple points of access and varying levels of security protocols, can create vulnerabilities in the trial’s data protection strategy. Ensuring consistent, high-level security across all systems becomes a complex task, increasing the risk of data breaches and compromising participant trust.
5. Cost Efficiency:
The operational costs associated with maintaining multiple point systems can be significant. Each system may require separate licensing fees, infrastructure, and support teams, leading to inflated operational expenses. Moreover, the effort to integrate these systems—or to manually consolidate data across them—further increases costs, without necessarily achieving the desired level of interoperability or efficiency.
Additionally, the inefficiencies and delays caused by point system limitations can extend trial timelines, leading to increased indirect costs, such as prolonged use of facilities or resources. These extended timelines can delay the time to market for new treatments, resulting in missed opportunities and financial losses.
The Platform Approach: A Comprehensive Solution
1. Integration and Interoperability:
Adopting a platform approach eliminates the challenges posed by information silos by providing a unified system that integrates data across all aspects of a clinical trial. This holistic view facilitates better decision-making, as all stakeholders can access comprehensive, real-time data. The platform’s interoperability ensures that new tools and technologies can be seamlessly incorporated, enhancing trial capabilities without disrupting existing workflows.
Furthermore, a unified platform fosters collaboration among trial stakeholders by providing a single source of truth for all trial data. This centralization simplifies communication and coordination, enabling more efficient trial management and quicker responses to emerging issues. The platform’s integrated analytics tools also allow for deeper insights into trial data, supporting more informed strategic decisions.
2. Scalability and Adaptability:
A platform solution is inherently designed to accommodate growth and change, whether related to trial size, geographic expansion, or regulatory updates. This scalability ensures that as a trial evolves, the management system can easily adjust to new requirements without the need for extensive modifications. The platform’s adaptability also supports the incorporation of innovative trial designs and methodologies, such as decentralized trials or adaptive trial designs, allowing research to stay at the forefront of scientific advancements.
The flexibility of a platform approach extends to global trials as well, with built-in features to manage linguistic and regulatory diversity across regions. This adaptability not only streamlines global trial management but also reduces the risk of compliance issues, as updates can be centrally deployed to accommodate new regulations or guidelines.
3. Enhanced User Experience:
A platform approach centralizes trial management into a single, user-friendly interface, significantly improving the user experience for participants, researchers, and sponsors. This consolidation simplifies training and reduces the likelihood of user errors, while also enhancing engagement and satisfaction among trial participants. The platform’s accessibility ensures that all stakeholders have easy access to the data and tools they need, supporting more efficient workflow and decision-making processes.
Moreover, the platform’s design often includes features specifically aimed at improving user engagement, such as mobile access, personalized dashboards, and user support. These features not only make the trial experience more pleasant for participants but also enhance the productivity and satisfaction of research teams and sponsors.
4. Compliance and Security:
A platform approach offers comprehensive compliance solutions that are regularly updated to reflect the latest regulatory changes. This centralization simplifies the task of maintaining compliance across all aspects of a trial, as updates are deployed uniformly across the platform. The unified nature of the platform also enhances data integrity and auditability, crucial factors in meeting regulatory requirements.
In terms of security, a platform solution can implement consistent, high-level security measures across all trial data and functionalities. This unified security strategy reduces vulnerabilities and simplifies the management of data protection protocols, ensuring the safety of sensitive participant information and maintaining trust in the trial process.
5. Cost-Effectiveness:
Consolidating trial management into a single platform can significantly reduce operational and maintenance costs. The elimination of separate licensing fees, infrastructure, and support requirements for multiple point systems results in direct financial savings. Additionally, the platform’s efficiency in managing trial data and workflows can lead to faster trial completion times, further saving resources.
The cost-effectiveness of a platform solution extends beyond operational savings, as the increased efficiency and adaptability can accelerate the time to market for new therapies. This quicker pathway from trial to treatment not only benefits patients but also offers financial advantages for sponsors through earlier revenue generation and competitive positioning in the market.
In conclusion, the shift from point systems to a platform approach in clinical trial management represents a critical evolution in the pursuit of medical research excellence. By addressing the limitations of traditional systems, platforms offer a path to more integrated, efficient, and effective clinical trials. As the healthcare landscape continues to advance, embracing these comprehensive solutions will be key to unlocking the full potential of clinical research.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com


