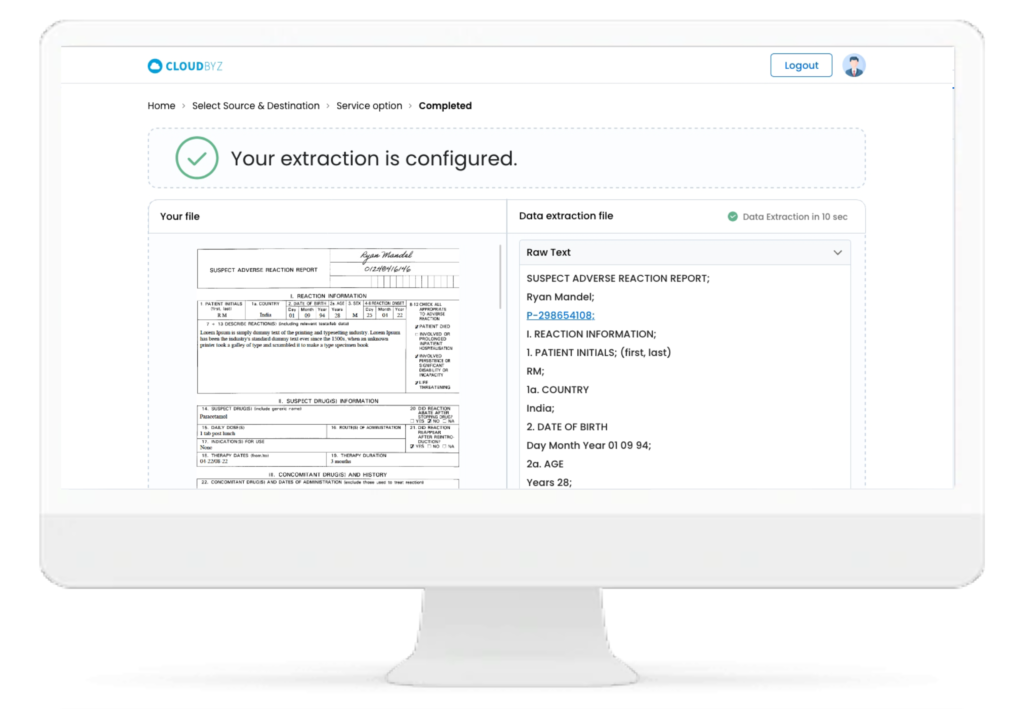
Introduction
Clinical research is a complex and highly regulated field where meticulous attention to detail is paramount. The process of building a clinical study can be both time-consuming and error-prone, as it involves extracting essential data from protocol documents and translating it into electronic data capture (EDC) systems like Cloudbyz EDC. Traditional methods of manual data entry and study build are not only resource-intensive but also prone to human errors. AI offers a groundbreaking solution to streamline this process, automating data extraction from protocol documents and ensuring faster, more accurate clinical study builds in Cloudbyz EDC.
Understanding the Role of AI
AI, powered by advanced machine learning algorithms, has the ability to understand and process natural language. It can read and comprehend complex documents, such as clinical trial protocols, and extract pertinent information intelligently. This technology can significantly enhance the efficiency and accuracy of the clinical study setup process in EDC systems like Cloudbyz EDC.
How AI Extracts Study Data from Protocol Documents
- Natural Language Processing (NLP): AI employs NLP techniques to analyze protocol documents. It recognizes key concepts, variables, inclusion/exclusion criteria, endpoints, and other critical information, even when presented in various formats or expressions.
- Contextual Understanding: AI not only identifies individual data points but also understands their relationships within the document. It discerns dependencies, hierarchical structures, and conditional statements, which are essential for accurate data extraction.
- Data Mapping: Once the relevant data is identified, AI maps it to the appropriate fields within the Cloudbyz EDC system. This mapping can be customized to align with the study’s specific requirements, making the process highly adaptable.
Benefits of Using AI for Data Extraction
- Speed and Efficiency: AI drastically reduces the time needed to extract data from protocol documents. What might take weeks through manual extraction can now be accomplished in a matter of hours or even minutes, accelerating the study build process.
- Accuracy and Consistency: Human errors are a significant concern in manual data extraction. AI ensures near-perfect accuracy, eliminating transcription errors and inconsistencies. This leads to higher data quality and more reliable clinical trials.
- Cost Reduction: By automating data extraction, organizations can reduce labor costs associated with manual data entry and minimize the need for extensive quality control measures. This cost-saving can be redirected towards other critical aspects of clinical research.
- Real-time Updates: AI can adapt to changes in protocol documents swiftly. When amendments or revisions occur, the AI can be retrained to recognize the updated information, ensuring that the study build remains current and compliant.
- Compliance and Audit Trail: AI maintains an audit trail of data extraction, providing transparency and accountability. This is crucial for regulatory compliance and audit purposes.
Implementation Challenges and Considerations
While AI offers transformative benefits, its successful implementation requires careful planning and considerations:
- Data Privacy and Security: Ensuring that sensitive patient data within protocol documents is handled securely and in compliance with data protection regulations (e.g., GDPR, HIPAA) is paramount.
- Validation and Training: AI models must be rigorously validated and continuously trained to adapt to evolving protocol document formats and language variations.
- Customization: The AI model must be customizable to accommodate different study designs, therapeutic areas, and data structures.
- Integration: Seamless integration with Cloudbyz EDC and other clinical trial systems is essential for a streamlined study build process.
Conclusion
AI has the potential to revolutionize the clinical study build process by automating data extraction from protocol documents. With its ability to process natural language and understand contextual nuances, it offers unparalleled speed, accuracy, and efficiency. Cloudbyz EDC, when integrated with AI, can enable researchers and organizations to accelerate their clinical trials, reduce costs, and improve data quality. As the field of clinical research continues to advance, embracing AI will be key to staying competitive and compliant in an increasingly complex regulatory landscape.
Cloudbyz EDC is a user-friendly, cloud-based solution that is designed to store and manage clinical data effectively throughout a clinical trial’s life cycle. Our innovative solution enables clinical research teams to efficiently collect, analyze, and manage clinical data of different complexity and size. Cloudbyz EDC is a scalable solution and meets all the essential regulatory compliance requirements such as FDA- 21 CFR Part 11, GCP, GAMP5, HIPAA, and EU- GDPR.
To know more about Cloudbyz EDC Solution contact info@cloudbyz.com


