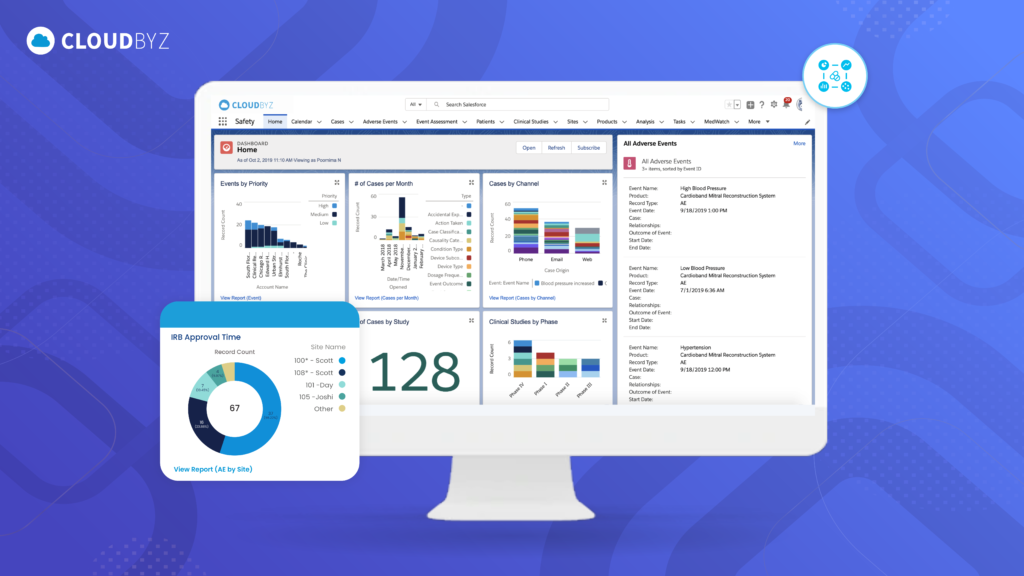
In the fast-paced world of life sciences, ensuring the safety and efficacy of drugs throughout their lifecycle is paramount. As the pharmaceutical landscape becomes more complex, the need for faster, more informed decision-making has never been greater. Real-time data access and analysis are transforming drug safety management by providing immediate insights that help organizations monitor, assess, and act on safety information promptly. This article explores the critical benefits of real-time data in drug safety management and how it leads to improved patient safety, regulatory compliance, and operational efficiency.
The Shift Towards Real-Time Data in Drug Safety
Traditionally, drug safety data collection and analysis have been slow and reactive. Pharmacovigilance teams would often rely on periodic safety reports, manual data aggregation, and delayed adverse event reporting. While this approach met regulatory requirements, it left room for delays in identifying and addressing potential safety risks.
Today, advances in technology and the digitization of healthcare have enabled real-time data collection, integration, and analysis. By leveraging these capabilities, pharmaceutical companies can monitor drug safety continuously, react swiftly to emerging safety signals, and make proactive decisions that mitigate risks.
Key Benefits of Real-Time Data in Drug Safety Management
1. Faster Signal Detection and Risk Mitigation
One of the most significant advantages of real-time data in drug safety management is the ability to detect potential safety signals quickly. In traditional pharmacovigilance, safety signals often take time to emerge due to the periodic nature of data collection and reporting. Real-time data access accelerates this process, enabling earlier identification of trends that could indicate an adverse event or safety concern.
- Proactive Signal Detection: With real-time data, safety teams can continuously monitor patient outcomes, adverse event reports, and clinical data to spot emerging trends. This proactive approach allows companies to intervene before a safety signal becomes a significant issue, reducing the risk of patient harm and regulatory action.
- Automated Monitoring: Advanced drug safety platforms leverage artificial intelligence (AI) and machine learning (ML) algorithms to analyze real-time data and detect abnormal patterns that may signal a potential safety concern. These automated systems reduce the manual workload on safety teams and ensure that no critical information is overlooked.
Example: If a new drug is showing a higher-than-expected rate of adverse reactions in a specific demographic, real-time data analysis can trigger an alert, prompting further investigation and risk mitigation strategies, such as revising dosage recommendations or updating safety labels.
2. Improved Decision-Making with Real-Time Insights
Real-time data equips decision-makers with up-to-the-minute insights into the safety profile of a drug. This enhanced visibility allows organizations to make more informed decisions regarding risk management, regulatory reporting, and product lifecycle strategies.
- Real-Time Dashboards: Drug safety platforms with real-time data capabilities often provide dashboards that display key safety metrics in real time. These dashboards offer an immediate view of adverse events, ongoing investigations, and overall drug safety performance, allowing teams to make quick, data-driven decisions.
- Dynamic Risk Assessments: As new data is collected, risk assessments can be continuously updated, reflecting the latest information. This dynamic approach ensures that decisions are always based on the most current understanding of a drug’s safety profile.
Example: During a post-market surveillance phase, real-time data could show an unexpected adverse event trend in a specific population. With immediate access to this information, the safety team can conduct a rapid benefit-risk assessment and decide whether additional risk mitigation actions, such as issuing a safety communication, are necessary.
3. Enhanced Regulatory Compliance
Maintaining compliance with regulatory requirements is a critical aspect of drug safety management. Regulators such as the FDA, EMA, and other global agencies require timely reporting of adverse events, periodic safety updates, and immediate notification of any emerging risks. Real-time data helps companies meet these regulatory demands more effectively.
- Automated Regulatory Reporting: With real-time data, organizations can automate the generation and submission of safety reports to regulatory bodies. This not only ensures timely reporting but also reduces the likelihood of errors that can occur with manual processes.
- Audit-Ready Documentation: Real-time data systems maintain a complete and up-to-date record of all safety-related activities. In the event of an inspection or audit, companies can provide regulators with immediate access to the latest safety information and documentation, demonstrating compliance with regulatory standards.
Example: A global pharmaceutical company managing multiple products across different regions can use real-time data to ensure that all safety reports are submitted according to each region’s regulatory timeline. Automated submissions based on real-time data reduce the risk of non-compliance and penalties.
4. Better Patient Outcomes through Continuous Monitoring
At the heart of drug safety management is the goal of ensuring patient safety. Real-time data enables continuous monitoring of a drug’s effects on patients, both during clinical trials and in post-market surveillance. This ongoing vigilance helps identify potential safety concerns as soon as they arise and allows for immediate corrective actions.
- Continuous Post-Market Surveillance: Once a drug is on the market, real-time data allows for continuous monitoring of adverse events, treatment efficacy, and overall patient outcomes. This is particularly important for detecting rare or long-term side effects that may not have been observed during clinical trials.
- Personalized Safety Monitoring: With the rise of personalized medicine, real-time data can be used to monitor drug safety at the individual patient level. This enables the detection of adverse reactions based on a patient’s unique characteristics, such as genetics or underlying health conditions.
Example: A patient enrolled in a clinical trial might exhibit early signs of an adverse reaction that could otherwise go unnoticed. Real-time data collected through wearable devices and other digital health tools can alert clinicians to intervene immediately, adjusting treatment plans and preventing more serious outcomes.
5. Operational Efficiency and Cost Savings
Real-time data not only improves patient safety and regulatory compliance but also drives operational efficiency. By automating data collection, analysis, and reporting, organizations can streamline their pharmacovigilance processes, reduce manual labor, and save costs.
- Streamlined Workflow: Real-time data systems reduce the need for manual data entry, aggregation, and analysis, allowing safety teams to focus on higher-value activities such as risk assessment and strategic decision-making.
- Cost Reduction: By detecting safety signals earlier and reducing the risk of large-scale recalls or litigation, real-time data systems can save companies significant costs associated with addressing safety issues after they’ve escalated.
Example: A pharmaceutical company using a real-time drug safety system could automate case management for adverse events, reducing the time and resources needed for manual data handling. This not only speeds up safety reporting but also cuts down on operational costs.
6. Collaborative Decision-Making with Cross-Functional Access
Real-time data systems provide a single source of truth accessible by multiple stakeholders within an organization. This cross-functional access enhances collaboration between safety, clinical, regulatory, and commercial teams, ensuring that everyone is working with the most up-to-date information.
- Cross-Departmental Collaboration: By providing real-time data access to various teams, drug safety solutions enable more effective collaboration between departments. Regulatory teams can ensure timely compliance, clinical teams can adjust trial protocols, and commercial teams can make informed decisions about product marketing strategies.
- Unified Data Access: A centralized real-time data platform ensures that all stakeholders have access to the same data, reducing miscommunication and ensuring consistent decision-making across the organization.
Example: A drug safety team monitoring real-time adverse event reports can instantly share critical findings with the clinical and regulatory teams, allowing them to adjust trial designs or submit expedited safety reports to regulators without delay.
Conclusion
In today’s dynamic pharmaceutical environment, real-time data is revolutionizing drug safety management by enabling faster signal detection, more informed decision-making, enhanced regulatory compliance, and better patient outcomes. The ability to access and analyze data as it becomes available allows organizations to move from reactive to proactive pharmacovigilance, ensuring that drugs remain safe and effective throughout their lifecycle.
Investing in real-time drug safety solutions is not just about meeting regulatory requirements—it’s about building a future-proof system that enhances patient safety, reduces operational inefficiencies, and strengthens overall product success. As technology continues to advance, real-time data will play an increasingly critical role in the evolution of drug safety management, shaping the future of how we monitor and protect public health.


