In 2024, the pharmacovigilance (PV) landscape continues to evolve rapidly, driven by the need for real-time data access, global regulatory compliance, and advanced risk management. As the life sciences industry faces increasing scrutiny and demands for greater transparency, choosing the right pharmacovigilance software has never been more critical. This comprehensive guide will walk you through the essential factors to consider when evaluating pharmacovigilance software and explain why Cloudbyz stands out as a top contender in this space.
1. Understanding Your Pharmacovigilance Needs
Before diving into software evaluation, it’s crucial to assess your organization’s specific pharmacovigilance needs. Consider the following questions:
- What is the volume of adverse events (AEs) your organization handles?
- Are you operating in multiple regions with different regulatory requirements?
- What level of integration do you need with existing clinical or safety systems?
- Do you require advanced signal detection and risk management capabilities?
- What is your organization’s scalability and future growth plans?
By clearly defining your needs, you can narrow down the software options that align with your organization’s goals and challenges.
2. Key Features to Look for in Pharmacovigilance Software
Once you’ve identified your needs, evaluate potential software solutions based on the following key features:
a) Regulatory Compliance and Global Reach
Why It Matters: Pharmacovigilance software must comply with global regulatory standards like E2B(R3), MedDRA coding, and GVP modules. It should also support regional requirements, such as FDA reporting in the U.S., EMA reporting in Europe, and other country-specific regulations.
Cloudbyz Advantage: Cloudbyz Pharmacovigilance is built with global compliance in mind. The platform supports E2B(R3) for seamless data exchange with regulatory bodies and adheres to MedDRA coding standards. Cloudbyz also automates the generation and submission of regulatory reports, ensuring compliance across different regions.
b) Comprehensive Case Management
Why It Matters: Effective case management is essential for tracking and resolving adverse events. The software should provide end-to-end case management, from data intake to final reporting, with automated workflows to enhance efficiency.
Cloudbyz Advantage: Cloudbyz offers a fully integrated case management system that supports the entire lifecycle of pharmacovigilance cases. Automated workflows ensure that cases move through the system efficiently, reducing manual intervention and minimizing errors.
c) Advanced Signal Detection and Risk Management
Why It Matters: Early detection of safety signals is crucial for preventing widespread issues. The software should offer advanced analytics and machine learning capabilities to identify potential risks and manage them proactively.
Cloudbyz Advantage: Cloudbyz Pharmacovigilance utilizes cutting-edge algorithms and machine learning tools for advanced signal detection and risk management. The platform enables users to identify trends and patterns in adverse event data, allowing for proactive risk mitigation.
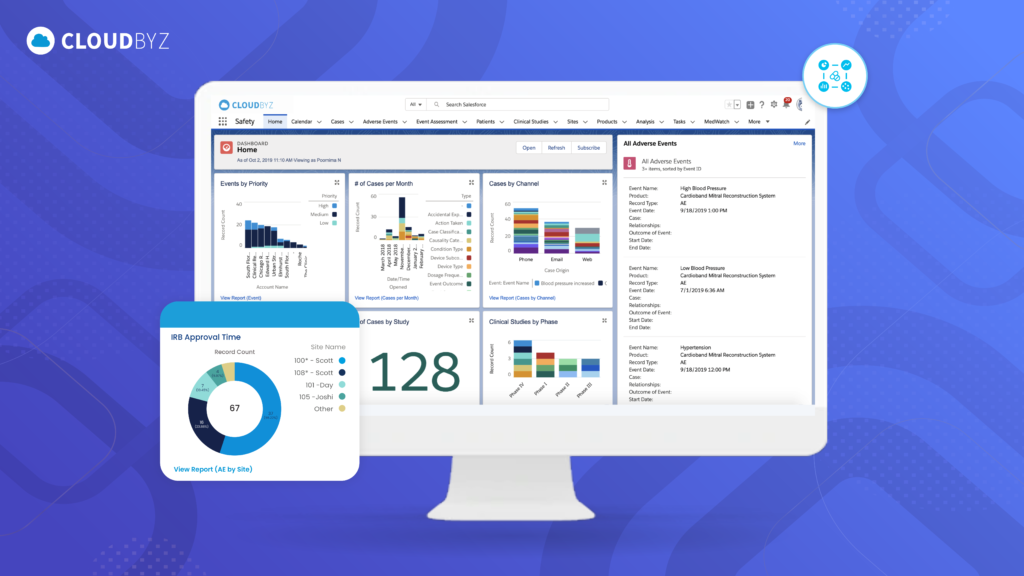
d) Real-Time Data Access and Reporting
Why It Matters: In a fast-paced regulatory environment, real-time access to data and the ability to generate reports quickly are vital. The software should provide intuitive dashboards and customizable reporting tools that allow users to access and analyze data on demand.
Cloudbyz Advantage: Cloudbyz offers real-time data access with powerful analytics and reporting tools. The platform’s interactive dashboards provide instant insights into safety data, and users can generate both standard and custom reports to meet regulatory and internal requirements.
e) Scalability and Customization
Why It Matters: Your pharmacovigilance needs may grow as your organization expands. The software should be scalable and customizable to adapt to increased data volumes, additional users, and evolving regulatory demands.
Cloudbyz Advantage: Cloudbyz is built on a scalable cloud-based platform that can grow with your organization. The platform is highly configurable, allowing users to customize workflows, reports, and integrations to meet specific needs without compromising performance.
f) Seamless Integration with Other Systems
Why It Matters: Pharmacovigilance software should integrate seamlessly with your existing systems, such as electronic data capture (EDC), clinical trial management systems (CTMS), and safety databases. This ensures data consistency and streamlines pharmacovigilance processes.
Cloudbyz Advantage: Cloudbyz offers seamless integration with various clinical and safety systems, including EDC, CTMS, and global safety databases like VigiBase and EudraVigilance. This interoperability ensures that safety data is consistent and accessible across different platforms.
g) User-Friendly Interface and Training
Why It Matters: A user-friendly interface is crucial for ensuring that all team members can efficiently use the software. Additionally, the availability of training and support resources can significantly impact the successful adoption of the software.
Cloudbyz Advantage: Cloudbyz features an intuitive user interface designed for ease of use. The platform provides extensive training resources and ongoing support, ensuring that users can quickly become proficient and fully leverage the software’s capabilities.
h) Data Security and Compliance
Why It Matters: Given the sensitive nature of pharmacovigilance data, robust data security and privacy measures are essential. The software should comply with regulations like GDPR and HIPAA, ensuring that patient data is protected at all times.
Cloudbyz Advantage: Cloudbyz prioritizes data security and privacy, complying with GDPR, HIPAA, and other relevant regulations. The platform employs advanced encryption, role-based access controls, and detailed audit trails to safeguard sensitive information.
i) Cost-Effectiveness
Why It Matters: While it’s important to invest in a robust pharmacovigilance system, the solution should also be cost-effective, offering a balance between functionality and affordability.
Cloudbyz Advantage: Cloudbyz offers a competitively priced solution that delivers high value for money. The platform’s modular pricing allows organizations to choose the features they need, ensuring that you only pay for what you use.
j) Vendor Reputation and Support
Why It Matters: Selecting a vendor with a strong reputation and a proven track record in pharmacovigilance is crucial. The software provider should offer reliable customer support and regular updates to keep the platform aligned with regulatory changes.
Cloudbyz Advantage: Cloudbyz is a trusted name in the life sciences industry, with a proven track record of delivering innovative pharmacovigilance solutions. The company provides exceptional customer support, regular software updates, and a strong commitment to helping clients achieve their safety and compliance goals.
3. Why Cloudbyz is a Top Contender for Pharmacovigilance Software in 2024
In a crowded market of pharmacovigilance software solutions, Cloudbyz distinguishes itself as a top contender for several reasons:
- Comprehensive Feature Set: Cloudbyz offers all the essential features you need in a pharmacovigilance solution, including advanced case management, signal detection, regulatory compliance, and real-time reporting.
- Scalability: Built on a cloud platform, Cloudbyz can easily scale to meet the needs of growing organizations, ensuring that your pharmacovigilance operations can expand without disruption.
- Ease of Use: With an intuitive user interface and extensive support resources, Cloudbyz ensures that your team can quickly adopt and master the platform.
- Integration Capabilities: Cloudbyz seamlessly integrates with other critical systems in your organization, providing a unified view of safety data across the board.
- Security and Compliance: Data security is a top priority for Cloudbyz, with robust measures in place to protect sensitive information and ensure compliance with global regulations.
- Cost-Effective: Cloudbyz offers a flexible, cost-effective solution that provides excellent value for money, making it an ideal choice for organizations of all sizes.
4. Steps to Implementing Cloudbyz Pharmacovigilance Software
If you’re considering Cloudbyz as your pharmacovigilance software solution, follow these steps to ensure a smooth implementation:
- Conduct a Needs Assessment: Evaluate your organization’s specific pharmacovigilance needs to determine which Cloudbyz modules and features are most relevant.
- Engage Key Stakeholders: Involve key stakeholders from different departments (e.g., clinical, regulatory, IT) in the selection and implementation process to ensure buy-in and collaboration.
- Develop an Implementation Plan: Work with Cloudbyz’s implementation team to develop a detailed plan, including timelines, milestones, and training sessions.
- Train Your Team: Leverage Cloudbyz’s training resources to ensure that all users are proficient in using the platform and understand how to maximize its features.
- Monitor and Optimize: After implementation, regularly monitor the system’s performance and work with Cloudbyz to optimize the platform as your needs evolve.
Conclusion
Choosing the right pharmacovigilance software is a critical decision that can significantly impact your organization’s ability to ensure patient safety and maintain regulatory compliance. By following this comprehensive guide, you can confidently evaluate potential software solutions and select the one that best meets your needs in 2024 and beyond.
Cloudbyz Pharmacovigilance emerges as a top contender, offering a robust, scalable, and user-friendly platform that addresses all the key requirements of modern pharmacovigilance. With Cloudbyz, you can ensure that your organization stays ahead in the rapidly evolving pharmacovigilance landscape, safeguarding patient safety and achieving regulatory excellence.


