Introduction
In the dynamic world of pharmaceuticals, ensuring the safety of drugs is a complex and ongoing responsibility. Pharmacovigilance, the science of detecting, assessing, and preventing adverse effects or any other drug-related problems, has traditionally been a reactive process, heavily reliant on manual data collection and analysis. However, the rise of data analytics has transformed pharmacovigilance into a more proactive and data-driven discipline, allowing for more informed decision-making and improved risk management. At the forefront of this transformation is Cloudbyz, a leader in Safety & Pharmacovigilance solutions, enabling companies to unlock actionable insights from their safety data.
The Evolution of Pharmacovigilance Through Data Analytics
The integration of data analytics into pharmacovigilance processes has fundamentally changed how drug safety is managed. With the ability to process and analyze vast amounts of data from various sources, pharmacovigilance professionals can now identify potential safety signals earlier and more accurately, leading to timely interventions and better patient outcomes.
- Big Data and Real-Time Monitoring:
- The sheer volume of data available in today’s digital age—ranging from electronic health records (EHRs) to social media posts—has introduced new possibilities for pharmacovigilance. Big data analytics allows for the real-time monitoring of adverse events, facilitating quicker responses to emerging safety concerns. This approach not only enhances the detection of rare adverse events but also improves the overall understanding of a drug’s safety profile.
- Predictive Analytics and Machine Learning:
- Predictive analytics and machine learning (ML) models are increasingly being used to anticipate potential safety issues before they manifest in larger patient populations. By analyzing historical data and identifying patterns, these models can predict the likelihood of adverse events, enabling pharmaceutical companies to take preventive measures and mitigate risks proactively.
- Integration of Diverse Data Sources:
- Data-driven pharmacovigilance goes beyond traditional safety data collection. By integrating data from clinical trials, post-market surveillance, patient registries, and even unstructured data from social media, companies can gain a comprehensive view of drug safety. This holistic approach helps in identifying correlations and trends that might be missed when analyzing data in isolation.
How Cloudbyz Safety & Pharmacovigilance Unlocks Actionable Insights
Cloudbyz has leveraged the power of data analytics to create a robust Safety & Pharmacovigilance solution that empowers organizations to derive actionable insights from their safety data. Here’s how Cloudbyz stands out in enabling data-driven pharmacovigilance:
- Centralized Data Management:
- Cloudbyz provides a centralized platform where all safety data—whether from clinical trials, post-marketing surveillance, or real-world evidence—can be aggregated and managed. This centralization ensures that all relevant data is easily accessible and can be analyzed collectively, leading to more comprehensive safety assessments.
- Advanced Signal Detection:
- With built-in advanced analytics tools, Cloudbyz enables more effective signal detection. The platform uses machine learning algorithms to scan large datasets, identifying patterns and anomalies that may indicate emerging safety issues. This capability allows companies to detect signals earlier and with greater precision, reducing the time it takes to address potential risks.
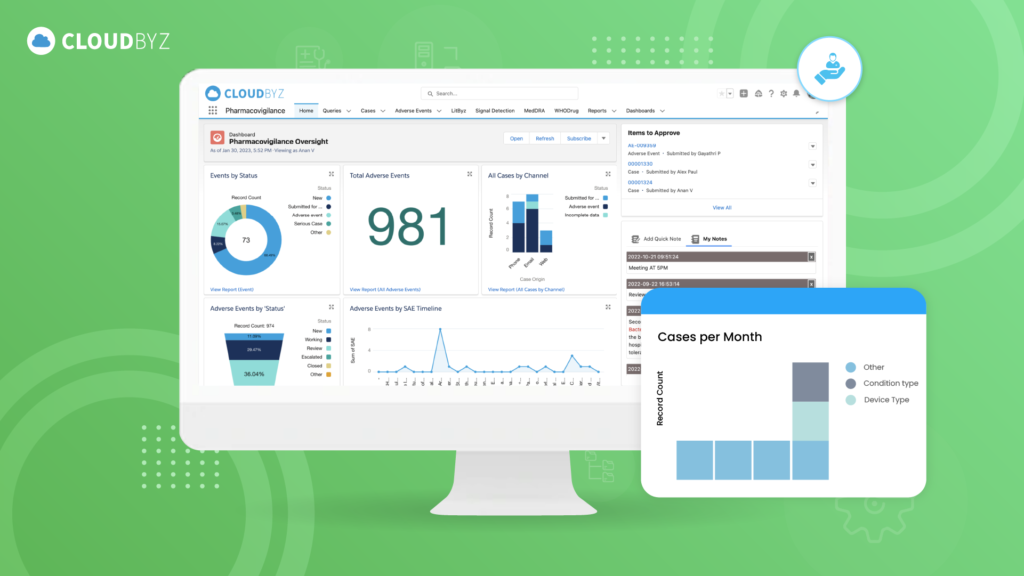
- Customizable Dashboards and Reporting:
- Cloudbyz offers customizable dashboards that provide real-time insights into pharmacovigilance activities. These dashboards allow users to monitor key performance indicators (KPIs), track adverse event trends, and generate reports tailored to specific needs. By presenting data in a visually intuitive manner, Cloudbyz helps stakeholders make informed decisions quickly and confidently.
- Integration with External Data Sources:
- The Cloudbyz platform is designed to integrate seamlessly with external data sources, such as EHRs, patient registries, and regulatory databases. This integration expands the scope of data that can be analyzed, offering a more complete picture of drug safety and facilitating better risk management strategies.
- Predictive Risk Management:
- Through the application of predictive analytics, Cloudbyz enables organizations to anticipate and mitigate risks before they escalate. The platform’s predictive models analyze historical and real-time data to identify potential safety concerns, allowing companies to take proactive measures to ensure patient safety and regulatory compliance.
- Automation and Workflow Efficiency:
- Cloudbyz automates many of the routine tasks involved in pharmacovigilance, such as adverse event reporting and regulatory submissions. This automation not only increases efficiency but also ensures that data is processed consistently and accurately. By reducing manual effort, Cloudbyz allows pharmacovigilance teams to focus on higher-level analysis and decision-making.
- Compliance with Global Regulations:
- Staying compliant with the ever-evolving global regulatory landscape is a significant challenge for pharmacovigilance teams. Cloudbyz simplifies this process by offering automated compliance features that ensure all activities align with regulatory requirements. The platform’s reporting tools are designed to meet the standards of various regulatory bodies, reducing the risk of non-compliance.
The Cloudbyz Competitive Advantage
In a crowded market, Cloudbyz distinguishes itself by offering a data-driven pharmacovigilance solution that is not only comprehensive but also highly adaptable to the needs of different organizations. The key features that set Cloudbyz apart include:
- Scalability:
- Whether it’s a small biotech startup or a large pharmaceutical corporation, Cloudbyz’s scalable platform can accommodate organizations of all sizes. The platform’s modular design allows companies to customize their pharmacovigilance processes according to their specific needs and resources.
- User-Friendly Interface:
- Cloudbyz prioritizes user experience, offering an intuitive interface that makes complex data analysis accessible to users at all levels. The platform’s user-friendly design ensures that pharmacovigilance professionals can easily navigate and utilize the system without extensive training.
- Real-Time Collaboration:
- In a globalized industry, collaboration across different teams and locations is essential. Cloudbyz facilitates real-time collaboration by allowing multiple stakeholders to access and work on the same data simultaneously. This feature enhances communication and ensures that everyone involved in pharmacovigilance activities is on the same page.
- Commitment to Innovation:
- Cloudbyz is committed to continuous innovation, regularly updating its platform to incorporate the latest advancements in data analytics and pharmacovigilance. This commitment ensures that Cloudbyz clients always have access to cutting-edge tools and technologies that keep them ahead of the curve.
Conclusion
As the pharmaceutical industry continues to evolve, data-driven pharmacovigilance will play an increasingly important role in ensuring the safety and efficacy of drugs. By leveraging advanced data analytics, companies can unlock valuable insights that drive better decision-making and more effective risk management. Cloudbyz Safety & Pharmacovigilance is at the forefront of this transformation, offering a platform that empowers organizations to harness the full potential of their safety data. With its centralized data management, advanced signal detection, and predictive analytics capabilities, Cloudbyz enables companies to take a proactive approach to pharmacovigilance, ensuring the highest standards of patient safety and regulatory compliance. In an industry where the stakes are high, Cloudbyz provides the tools and insights needed to navigate the complexities of modern pharmacovigilance successfully.


